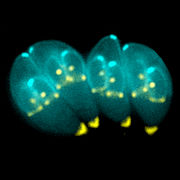
Unicellular parasites that reside and multiply inside the cells of their hosts must be adapted to penetrate the host cell without killing it and then to escape once they have multiplied or completed the necessary development. The method and timing of this exit from a host cell are crucial aspects of the life history strategy of these parasites. Toxoplasma gondii, one of the most ubiquitous parasites in the world, is being used to investigate the triggers that initiate this breakout.
Toxoplasma can infect almost all birds and mammals, including humans; and estimates suggest one third of the human population have been exposed to, or harbour, infections. Approximately 350,000 people are infected annually in the UK and 60 million in the USA. Infection can occur by ingesting soil, water or vegetables contaminated with cat faecal material containing oocysts of the parasite, or by eating raw or undercooked meat that contains Toxoplasma tissue cysts. Initial infection may cause mild flu-like symptoms but most people are unaware that they are infected as the parasite is usually kept in check by the immune system. However, it can cause serious problems where the immune system is compromised or diminished as with pregnant women, those with AIDS, and new born infants who have been infected in the womb (a process known as congenital transmission).
Although the parasite divides asexually in any warm blooded animal, it can only reproduce sexually in the cat. If the cat has eaten anything containing cysts of the parasite (for instance an infected mouse) the parasite will invade the epithelial cells lining the gut, undergo sexual reproduction here and produce oocysts that are shed with the faeces. These can survive in the environment for many months. If the oocysts are accidentally ingested by humans or other animals the cyst wall dissolves and parasites are released to invade the intestinal cells, where they reside in a membrane bound compartment, the parasitophorous vacuole. Here they change into a form called a tachyzoite and begin to divide and multiply rapidly.
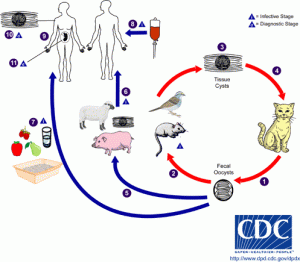
They exit this cell and move into the blood stream where they are disseminated around the body, invading organs, including the brain. Eventually the immune system causes them to change to a semi-dormant form enclosed in a cyst that is barely visible to the human eye. Cysts predominantly form in the cells of the brain or the muscles. If this infected tissue is eaten these cysts will give rise to a new infection.
A key component of the success of this parasite is its ability to rapidly reproduce itself in the protected environment of the parasitophorous vacuole and then for its progeny to escape from the membranes of the vacuole and that of the cell itself, and to invade more cells. One of the intriguing questions that arises from our knowledge of this process is – what is the trigger that initiates this process of escape? Marijo Roiko, Nadezhda Svezhova and Vern Carruthers from the University of Michigan Medical School, USA, have been investigating this question.
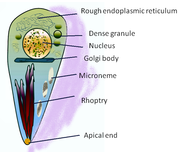
Two events key to the escape of the tachyzoites are the initiation of motility and the secretion of proteins from organelles called micronemes (see the cartoon of tachyzoite structure). These molecules have lytic properties to break down the membranes that confine the parasites and adhesive properties that probably help the parasites stick to new host cells before invasion. It was previously known that the tachyzoites required an acidic environment to stimulate their motility, whereas potassium ions in the medium surrounding them suppress motility.
The group from Michigan Medical School cultured T. gondii in human foreskin fibroblasts. Initially they confirmed the importance of a low pH; it stimulated movement, induce egress of parasites from the host cell and induced microneme secretion, even if the concentration of potassium ions remained high. By monitoring the pH in the parasitophorous vacuole during parasite development they showed that the pH of the parasitophorous vacuole only decreases late in the cycle of parasite replication (before parasite egress). This implied a role for an active proton flux to generate the trigger for microneme secretion and initiation of motility prior to exiting the cell. The researchers suggested that P-type ATPases, located on the parasite membrane, may pump protons from the parasite cytoplasm into the parasitophorous vacuole. Over time this would give rise to the build-up of an acidic environment surrounding the tachyzoites.
They next turned their attention to a microneme protein called perforin-like protein 1 (PLP1) that had previously been shown to be involved in damage of the host cell membrane during parasite egress. Genetically modified parasites that had the plp gene knocked out were treated to induce microneme secretion. The secretions from these parasites without PLP1 activity did not cause membrane damage. PLP1 activity was suppressed at a neutral pH, its activity peaking at pH 5.4. In fact, both the membrane binding and the lytic activity of PLP1 increased in an acid pH. PLP1 also acted on the membranes of uninfected host cells, possibly aiding invasion by the newly released tachyzoites. However, although low pH increased binding to new cells, it also increased PLP1 dependent cell wounding.
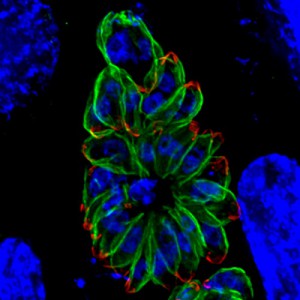
Marijo Roiko and colleagues propose a working model for the process of the egress of Toxoplasma tachyzoites from a host cell. Initially motility, microneme secretion and PLP1 activity is supressed by the high potassium ion concentration in the parasitophorous vacuole. Eventually acidification of the vacuole by protons from the replication tachyzoites acts a primary trigger that overcomes this suppression the parasites become motile and microneme secretion begins. The damage to the membrane of the parasitophorous vacuole caused by the secreted PLP1 will allow leakage of potassium ions. This would promote calcium (Ca+2) signalling that would, in turn, promote motor activation and more microneme secretion, thus enhancing parasite egress from the cells. All this would only occur once the parasites had replicated inside the original host cell. When the tachyzoites have escaped from their host cell the neutral pH of the surrounding environment would suppress the lytic activity of PLP1 and wounding of newly to be infected cells would be avoided so that the parasitophorous vacuole of the new host cell could be formed. They point out that this model does not exclude the existence of other levels of regulation of egress.
This is clearly a simple yet elegant way to trigger cell exit once replication occurs. I am left wondering if similar strategies are employed by other intracellular parasites that have to negotiate their way through multiple membranes before they can invade further cells.

Comments