You don’t need to be a peasant from the 14th century to be afraid of the plague. Although most famous for wiping out swathes of the world population in antiquity, the ‘Black Death’ never disappeared. The causative agent, a bacterium called Yersinia pestis, continues to cycle around in its wild hosts. Occasionally, it will strike humans again, causing infection outbreaks globally, such as those in Madagascar in 2017. Given its historical importance, and the ongoing threat to public health, it may surprise readers that much about the pathogen’s spread remains a mystery.

Plague is spread by fleas carried by rodents, however this process alone is not sufficient to explain how Yersinia can maintain itself in the wild between epidemics. For one thing, Y. pestis escapes the flea midgut by inducing the bug to vomit. Unlike malaria, the organism does not spread to the salivary glands, so it needs to violently force its own way out of the digestive system. The oft-demonised flea is an unwilling participant in this method, and transmission is not only inefficient but also damages the flea. A key part of the puzzle is missing – around half of Europe perished to a disease that spreads faster than should be possible.
A team of researchers at the University of Missouri and University of Central Missouri suspected that there must be another way that Y. pestis moves within, and between, fleas. With a modified bacterial isolate that emitted fluorescence, they fed infected blood to tropical rat fleas (Xenopsylla cheopis – the vector blamed for the Black Death itself) and looked within their tiny bodies to find where the pathogen ended up. As shown in their pre-print, growths in the midgut were found, as expected, confirming the frightening swift growth of the bacteria upon uptake (developing within a few days).
However, for the first time, they also saw their glowing cells in a new tissue of the flea – the reproductive organs. Female ovaries and male testes fluoresced with bacterial colonies. Following this up with electron microscopy demonstrated bodies, not found in uninfected entities, consistent with bacterial inclusions. They even developed a beautiful image of the Yersinia cells dividing in the X. cheopis ovaries. This tantalised a new method of transmission previously undescribed, as well as granting a new meaning to the unfortunate typo Y. testis.
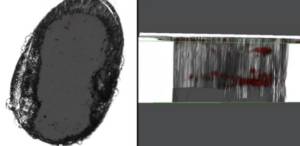
Critically, when these fleas reproduced, the Yersinia persisted into their progeny. The fluorescing bacteria showed up in the eggs and the larvae of infected adults, and when they grew into adults themselves their midguts came pre-infested with Y. pestis. Are these bacteria accidental passengers, or are they capable of further infection? The experimenters measured how well the isolated bacteria could kill cells in a petri dish, finding that they were competent at propagating the threat, albeit at low levels. Now, finally, a key missing step of the life cycle of plague bacteria may have been identified.
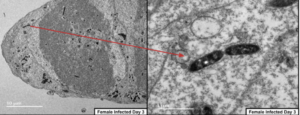
With healthy scientific scepticism, the Missourian team further proved that the Y. pestis was not being transferred to progeny via the regurgitations of the adults. They isolated eggs from infected parents and grew them up in a sterile environment. With no other method of getting infected, when these eggs hatched and grew into larvae harbouring Yersinia, it provided conclusive evidence of vertical, transovarial transmission. However, the fact that so few experiments were conducted with the vertically-infected generation of fleas themselves suggests that there were not enough alive for follow-up, begging the question of whether these progeny are encountering unfortunate side-effects of the bacterial infestation.

Although we may now possess a new piece of the puzzle, there are many adjacent sections still missing. For one, there is no confirmation that the bacteria transmitted to flea eggs can then be transmitted to a rodent, or indeed a human, host. Furthermore the mechanism behind this method of transmission needs to be resolved. How does Y. pestis traverse from the midgut to the reproductive organs? Can Y. pestis be transmitted by both infected eggs and sperm, or only one of the gametes? Additionally, this publication is a pre-print, meaning that it has yet to go through peer-review, a key element of disseminating robust scientific results.
Verifying these results could provide a breakthrough in understanding an ancient disease.

Comments