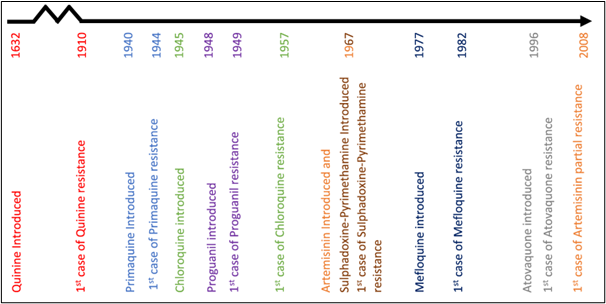
Antimalarial drug use on a mass scale
In June 2022, the WHO released new guidelines for the treatment and prevention of malaria. These updated guidelines are less restrictive on drug use, suggesting that antimalarial drugs will be used more often on a ‘mass scale’. A summary of these changes can be found here.
Antimalarial drug use is likely to increase following these recommendations. As antimalarial drug use increases, the selection pressure for the evolution of drug resistance also increases. Therefore, it is important to understand how this could impact the evolution of drug resistance. A recent systematic review, published in Malaria Journal in 2022, aimed to find out. The researchers focused on the mass use of the drug Dihydroartemisinin-Piperaquine (DHA-PPQ), which is a popular option for mass drug administration.
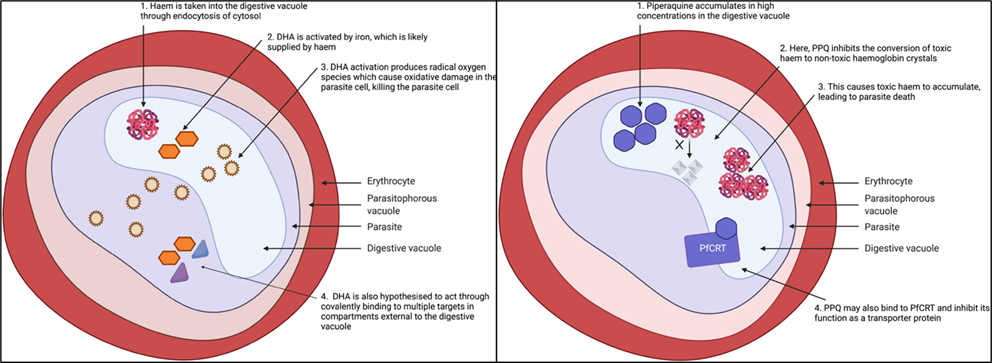
How does DHA-PPQ work?
Dihydroartemisinin-piperaquine is drug with two components: fast-acting dihydroartemisinin, and slow acting piperaquine. The fast-acting dihydroartemisinin has a short half-life and kills parasites quickly. This is paired with slow acting piperaquine, which ‘mops up’ by killing any parasites that are still remaining. The images below show how each of these components is hypothesised to work. This drug is an effective antimalarial, but resistance has been found in Southeast Asia.
Monitoring resistance to DHA-PPQ
Monitoring the evolution of drug resistance can be done in different ways. The gold standard is to use ‘therapeutic efficacy studies’ in a clinical setting. The WHO standard protocol for these studies involves a diagnosis of malaria, usually with microscopy, and then supervised use of a particular drug, and follow-up of the patient for a certain number of days. The recommended follow up time to measure therapeutic efficacy of DHA-PPQ when treating falciparum malaria is 42 days, which should detect 95% of clinical failures. So, this is a very robust, but very time-intensive and logistically challenging way of monitoring resistance.
To supplement these therapeutic efficacy studies, researchers can monitor genomic changes in the parasite DNA, termed ‘molecular markers’. These markers are genomic mutations which have previously been associated with resistance to antimalarial drugs. Monitoring molecular markers is particularly useful on a population-based level. For example, blood samples or finger-prick dried blood spots can be sampled from people living in malaria endemic areas. The parasite DNA can then be extracted from these blood spots, and the parasite DNA can be sequenced. Molecular markers associated with resistance can then be identified. By monitoring the prevalence of these molecular markers on a population level, researchers can provide early warning signals to WHO or National Malaria Control Programmes, if the prevalence of a marker associated with drug resistance in an area is changing.
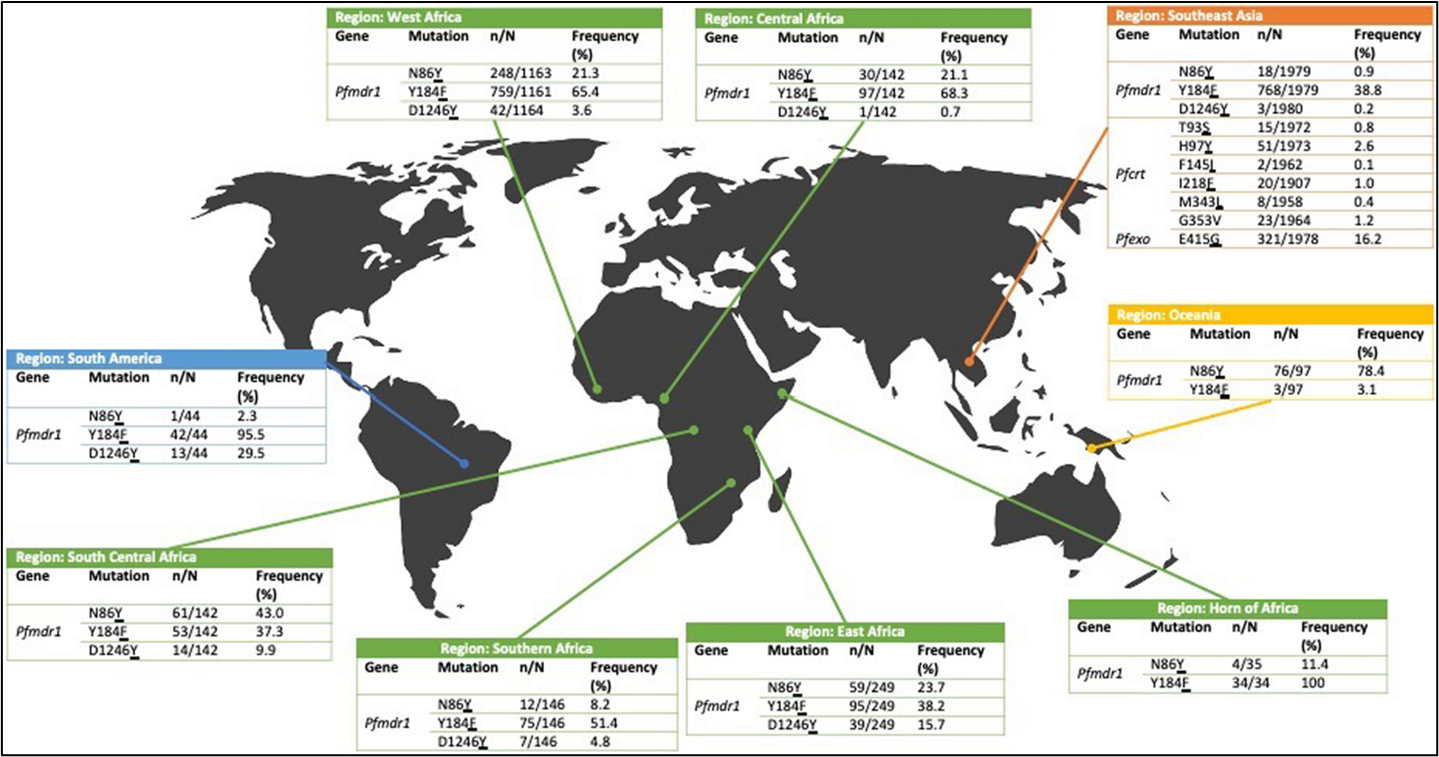
In their review, Moss et al., 2022. calculated global frequencies of molecular markers previously hypothesised to be associated with resistance to DHA-PPQ. They did this using open-source whole genome sequence (WGS) data for Plasmodium falciparum parasites. These frequencies can be seen in the map below. Importantly, these markers are not perfect, as they are not always associated with DHA-PPQ resistance. Therefore, it is likely that there are other genetic changes which lead to DHA-PPQ resistance which need to be identified.
How has mass use of DHA-PPQ impacted molecular markers of resistance?
There is an opportunity to measure molecular markers of resistance to provide early warning signals of resistance evolution. To facilitate this, researchers need to measure the prevalence of molecular markers before and after the implementation of antimalarial drugs. More widespread adoption of this approach would increase our understanding of how mass use of antimalarials is impacting resistance evolution. Moss et al., 2022. conducted a systematic review of studies which used DHA-PPQ on a mass scale. The review found that only 20 out of the total 96 studies measured and reported on the prevalence of molecular markers associated with drug resistance.
Increased genomic capacity is essential
This systematic review concluded that a greater focus and more investment is needed on genomic surveillance in trial and programmatic settings. This would enable broader scale monitoring of molecular markers associated with resistance. In turn, this would allow the research community to better understand the impact that mass antimalarial use has on the evolution of drug resistance. This is increasingly important, as mass use of antimalarial drugs is likely to increase in the future.

Comments