This blog post is part of a series featuring new articles published in the LCNTDR Collection: Advances in scientific research for NTD control, led by the London Centre for Neglected Tropical Disease Research (LCNTDR). Stay tuned for updates on Twitter @bugbittentweets and @NTDResearch. You can find other posts in the series here.
Soil-transmitted helminths (STHs) are a group of parasitic worms that infect humans, causing a wide spectrum of disease and morbidity in their hosts, notably anaemia, growth retardation, and delayed cognitive development in children. Approximately 1.5 billion people are currently thought to be infected with STHs worldwide.
Anthelminthic (deworming) drugs such as albendazole are effective at reducing worm burdens in individuals, but large-scale programs have generally failed to bring about elimination of STH infestation at the population level (check out this previous Bugbitten blog to find out more about global efforts to reduce STHs). Indeed, the World Health Organization targets morbidity control (keeping the prevalence and intensity of infections low) in children and women of child-bearing age over attempts to break transmission and eliminate.
The DeWorm3 trial (DW3), however, aims to test the hypothesis that frequent (twice a year) mass drug administration (MDA) with high coverage (>90%) can break the transmission of the parasites over a relatively short period of three years. If successful, this strategy would offer a clear endpoint to the current cycle of large-scale morbidity-controlling drug administration. DW3 is a cluster-based randomized trial that compares intense 6-monthly MDA with country-specific standard of care, and takes place at sites in India, Benin and Malawi. The primary aim of the trial is to bring STH prevalence to below 2% through MDA as an indicator of elimination.
The purpose of our study was to use data collected at the baseline of the study to forecast the results at the DW3 trial endpoint and also to predict the longer-term impact of the trial. Baseline data was used to infer parameter values (using a Bayesian approach) for a parasite transmission model, independently for each cluster in each country of the study.
The parameters fitted represent the intensity and variability of contact between individuals, processes that are most likely to vary across different communities and between countries. The fitted parameters were then used in a simulation of the trial, including the rounds of MDA and the standard of care interventions in both the control and intervention arms and diagnostic sampling of the trail population at the endpoint.
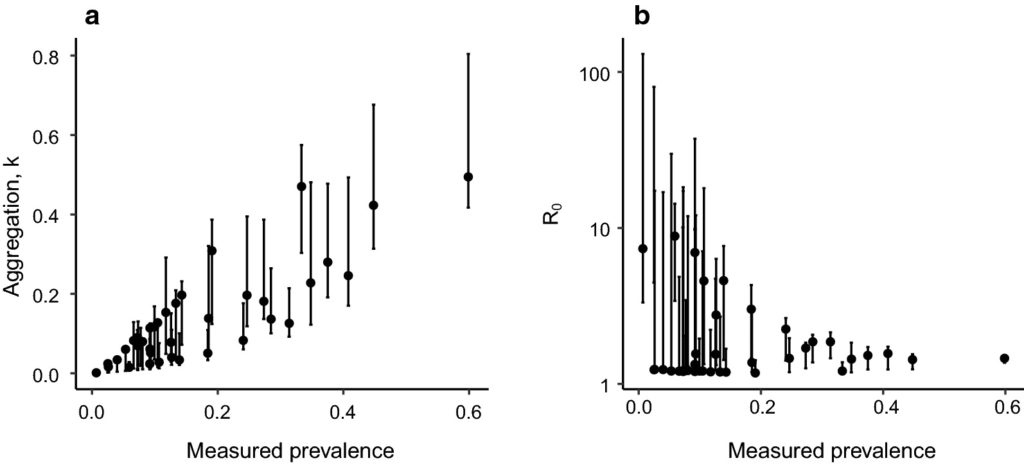
Results from the fitting process and the simulations emphasise the importance of cluster-level variability in the transmission of parasites on the results of the trial. Parameter fits to baseline data indicate a wide range of transmission intensities and degrees of parasite aggregation in the population among clusters, as shown in this graph.
Overall, the primary goal of the DW3 trial is predicted to be met; that is, the reduction of prevalence in the intervention arms of the study to less than 2%. However, variability in transmission intensity and parasite aggregation across clusters leads to a range of responses among clusters to the 6 rounds of MDA. The graph below shows that, while some clusters are predicted to be cleared of parasites, others are much less effected. The degree of impact is clearly not directly related to the baseline prevalence of parasites in a cluster.
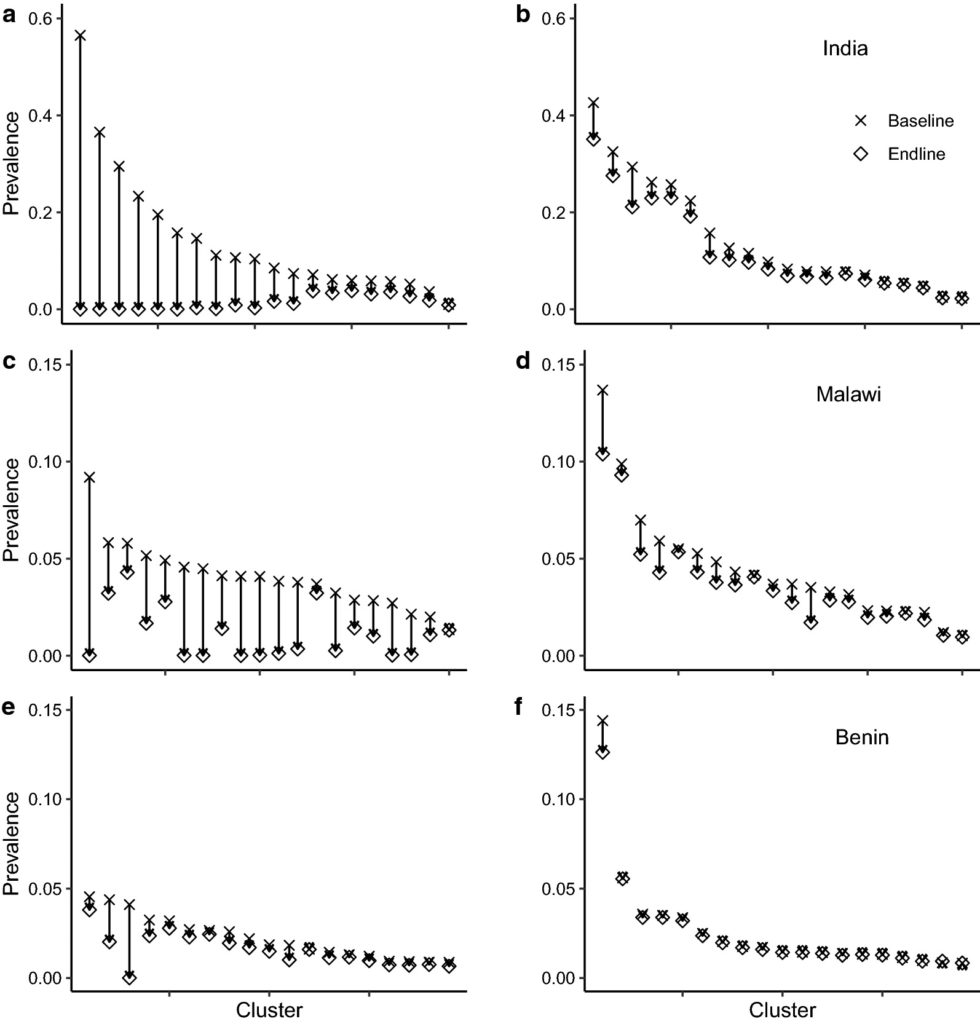
After the trial ends, the simulation predicts that clusters where transmission has not been interrupted will experience a ‘bounce-back’ in their parasite prevalence. Hence, the application of high coverage, highly uniform, MDA may result in the generation of localised “hot-spots” of parasite transmission among parasite-free areas within a treated region.
It must also be recognised that the parasite transmission model underlying the simulation contains assumptions which have yet to be tested, given the absence of appropriate data from the field. An important assumption is the detail of who infects whom during transmission events, which will be determined by patterns of behaviour and social structure (for example, household structure).
When DW3 data is fully available, they will provide invaluable information to modellers on the nature of parasite transmission, particularly at low prevalence, which will greatly strengthen the ability of models to forecast the effects of MDA in future.
 The study featured in this blog post was published in the LCNTDR Collection: Advances in scientific research for NTD control, led by the London Centre for Neglected Tropical Disease Research (LCNTDR). The collection has been publishing in Parasites & Vectors since 2016, and releasing new articles periodically. This series features recent advances in scientific research for NTDs executed by LCNTDR member institutions and their collaborators. It aims to highlight the wide range of work being undertaken by the LCNTDR towards achieving the United Nations Sustainable Development Goals as well as supporting the objectives of the World Health Organization road map for neglected tropical disease 2021-2030.
The study featured in this blog post was published in the LCNTDR Collection: Advances in scientific research for NTD control, led by the London Centre for Neglected Tropical Disease Research (LCNTDR). The collection has been publishing in Parasites & Vectors since 2016, and releasing new articles periodically. This series features recent advances in scientific research for NTDs executed by LCNTDR member institutions and their collaborators. It aims to highlight the wide range of work being undertaken by the LCNTDR towards achieving the United Nations Sustainable Development Goals as well as supporting the objectives of the World Health Organization road map for neglected tropical disease 2021-2030.
The LCNTDR was launched in 2013 with the aim of providing focused operational and research support for NTDs. LCNTDR, a joint initiative of the Natural History Museum, the London School of Hygiene & Tropical Medicine, the Royal Veterinary College, the Partnership for Child Development, the SCI Foundation (formerly known as the Schistosomiasis Control Initiative) and Imperial College London, undertakes interdisciplinary research to build the evidence base around the design, implementation, monitoring and evaluation of NTD programmes.
You can find other blog posts in the series here.

Comments