
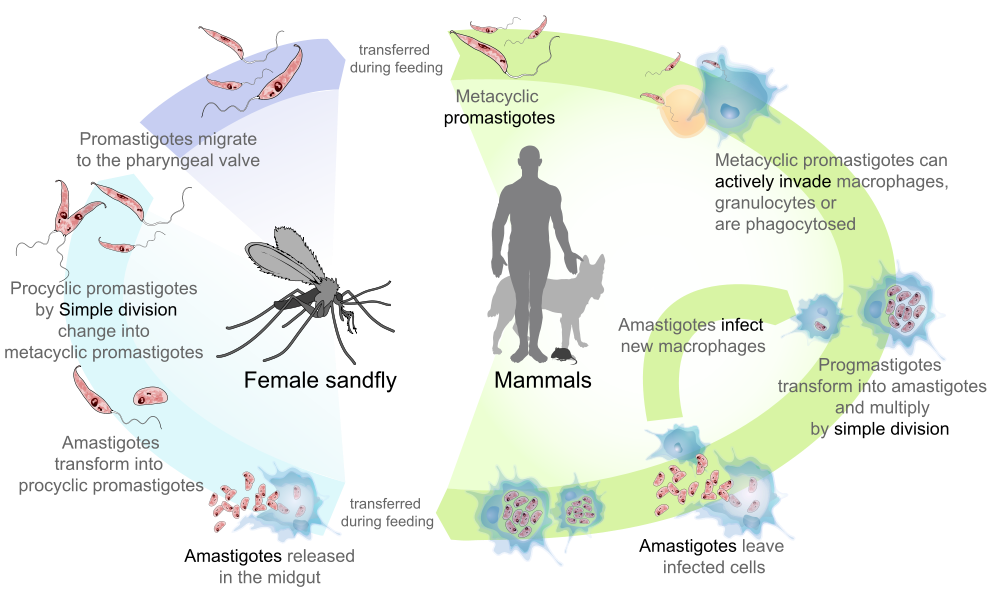
Within the sand fly, Leishmania transform into a number of flagellated promastigote forms, each with specific roles to play in colonising the vector for onward transmission. However, to survive in a sand fly is not straightforward.
The problem
As Leishmania live exclusively in the sand fly gut this can present some unique problems for the parasite. Firstly, they need to resist the hostile proteolytic environment of the bloodmeal as it is digested and survive assault from oxidative radicals. Next, they have to get out of the digested bloodmeal, which is encased in a chitinous peritrophic matrix, before the sand fly defecates. Thirdly, they must resist being lost from the sand fly when it defecates and find their way to the sand fly mouthparts to develop into its infectious form, the metacyclic promastigote. Here they face the final challenge of making their way into the vertebrate host’s skin against the flow of an incoming meal of fresh blood without being swept back into the midgut.
Midgut attachment
One of the most critical steps to survive in a sand fly is to resist being defecated with the digested bloodmeal.
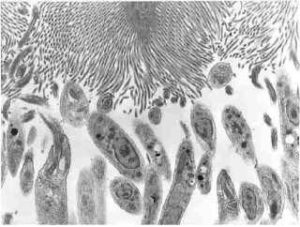
To do this Leishmania anchor themselves between the microvilli that line the midgut in a process that involves the parasite ‘adhesin’, lipophosphoglycan (LPG). This glycolipid coats the entire surface of promastigotes, which undergoes significant modification when the parasites differentiate into the infectious metacyclic promastigote form. This prevents re-attachment to the gut, giving them the best chance of being transmitted.
Epidemiologically, sand flies fall into two categories – they are either restrictive or permissive. In restrictive sand fly species, attachment is provided by LPG of nectomonad and leptomonad promastigotes in the early- to mid-phase of Leishmania development binding to a gut expressed lectin; resulting in the selective transmission of only one Leishmania species. In contrast, most sand flies are more permissive and can host a wide range of Leishmania species through an unknown mechanism. However, one clue to their attachment stands out – permissive sand flies line their gut with a mucus which contains an abundance of a particular sugar, N-acetyl galactosamine (GalNAc).
Atomic Force Microscopy
Our collaborative work between parasitologists, biophysicists and synthetic chemists, published in the Royal Society of Chemistry, explains how force spectroscopy was used to probe the surface of nectomonad and metacyclic promastigotes of Leishmania mexicana with tips coated with a GalNAc mimic. We measured Leishmania adhesion by using an atomic force microscope (AFM), able to measure adhesion of a few picoNewtons (pN). The AFM contains a tiny tip that is attached to its controller via a cantilever support.
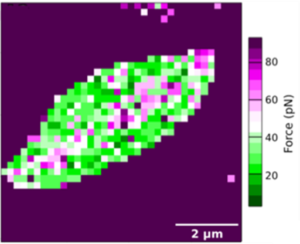
When the tip makes contact with a surface, the cantilever bends a little. This bending can be measured, and from this the force of interaction calculated and a nanoscale map of surface adhesion can be constructed for each parasite.
Using AFM, we tested the hypothesis that LPG may still be involved in attachment to permissive sand fly guts through glycan-glycan interactions – i.e. not involving lectins. We found that there was direct interaction between this GalNAc and LPG which was restricted to the gut-adherent promastigote stages and could be blocked by the introduction of excess GalNAc. This new mode of binding is comparable to the attachment of pili of gut-pathogenic bacteria to the mucus of human intestinal epithelia and offers a new model to study the competency of sand flies for Leishmania and their transmission.
Transmission blockade
Data collected from these experiments and previously published studies on Leishmania infections in sand flies allowed us to mathematically model the likely performance of a form of a transmission-blocking vaccine based on this mechanism. We found that such a vaccine could perform well, although if the vaccine were not effective enough it would exacerbate the problem. Further research is required to tease this relationship apart in order to direct the search for the most appropriate vaccine candidates.
A new hypothesis for the competency of permissive sand fly vectors?
As yet, it is unclear how much midgut adhesion contributes to the vector competency for Leishmania transmission. But the discovery that Leishmania can use their LPG to directly bind to sugars is a big step forward in our understanding of the interaction between Leishmania parasites and sand flies and, possibly, the vertebrate host following transmission.
Looking to the future, what excites us the most is the enormous potential biophysics and interdisciplinary research has to offer to explore vector-parasite and vector-parasite-host interactions.

Comments