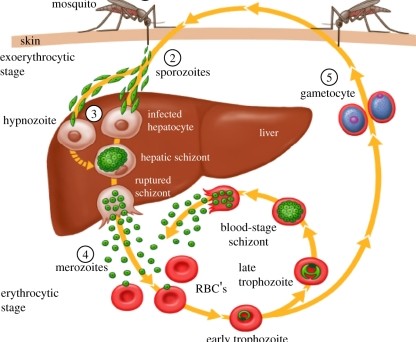
A well-known symptom of malaria is the cyclical wave of fever and shaking chills that is repeated in multiples of twenty-four-hour periods. These fevers and chills occur when infected red blood cells rupture synchronously, releasing the merozoite stages of the parasite, which then invade new red blood cells and begin another cycle of replication, beginning as a ring stage. The parasite’s inter-erythrocytic developmental cycle is following a circadian rhythm.
Circadian rhythms
Cyclical patterns of behaviour or physiology based on daily cycles are very common in nature and are known as circadian rhythms. They are usually regulated by biological oscillators, known as circadian clocks. They are responsive to both endogenous processes and abiotic factors in the environment which are normally synchronised. Internal rhythms are maintained by a subset of core clock genes, such as the Period genes (Per), that operate via a transcription-translation negative feedback loop (TTTL) regulated by their protein product.
The evolutionary importance of the coordination of parasite and host circadian rhythms is gaining recognition, and coordination between the malaria parasite and its host is no exception. Sarah Reece and her group at Edinburgh University demonstrated a decade ago that disruption of this cyclical process is costly for both the survival of the malaria parasite in its mammalian host and its transmission to mosquitoes. Using a mouse malaria, Plasmodium chabaudi, they showed that distinct stages of parasite progression during its asexual replication within the erythrocyte, coincided with the feeding rhythm of its nocturnal mouse host. The parasites remain in the early developmental ring stage when the mouse is fasting (daytime) and complete their development at the end of the hos- feeding phase. But what drives the synchronous development of the parasite and ties their development cycle to host circadian rhythms?
What drives malaria circadian rhythms?
The malaria parasite has an intrinsic clock, but no clock genes have been identified in the genome. Could host circadian rhythms be driving the parasite’s circadian cycle?
A study published in 2018 described how changing the feeding regime of nocturnal mice to daytime feeding desynchronised the rhythmic output of the light-entrained region of the hypothalamus and a peripheral oscillator located in other tissues. When these mice were infected, the timing of the parasite’s erythrocytic cycle was inverted compared with night-fed mice, but it returned to normal when night feeding was restored. This suggested host circadian rhythms associated with feeding patterns may indeed be driving the parasites’ circadian rhythm.
In the Edinburgh group’s recent publication, they have come to the same conclusion. They set out to determine whether the parasite’s erythrocytic cycle matches a mouse rhythm that is driven by a host TTTL, or whether the cycle is driven a peripheral oscillator associated with host feeding.
The investigation
Experiments were either conducted with wild type (WT) mice or with Per1–Per2 double knockout mice that do not exhibit circadian rhythms of locomotor activity and body temperature. Mice were either given continuous access to food, or food was restricted to the daytime, in conflict with their normal feeding pattern.
Initially, all mice were infected with parasites that were in different stages of development (desynchronized) to determine whether synchrony would be established in any of these four situations. By 4-5 days post-infection parasites in all four groups had become synchronised, however, parasites in the Per1/2-null mice that were fed ad libitum had a severely dampened rhythm.
Parasites that were all at the ring stage (synchronous) were then used to infect WT mice and Per1/2-null that were fed during the day and Per1/2-null mice that were fed ad libitum. Parasites rescheduled their developmental cycle to coincide with the new host feeding rhythm. As before, the amplitude of synchrony of ring stage parasites was over 50% higher in mice on a feeding regime than those fed ad libitum. Timing of the cycle also differed according to the feeding regime. Ring stages peaked 4h after the end of the day-feeding period in the time-scheduled feeding groups and 8h earlier in the ad libitum group.
These experiments clearly demonstrated that parasite synchrony and timing is not driven by host cycles controlled by a core-TTTF clock, but that the host feeding pattern is involved in both timing of the cycle and synchronization of it.
Ad libitum feeding
The authors discuss various reasons that might explain why the initially synchronous parasites in the clock-disrupted, ad libitum fed, mice did not become completely desynchronized. They suggest that a product of food digestion, such as glucose, is a likely signal to give the parasites a clue to timing, but the interplay of host rhythms that could affect development timing, parasite fitness and disease severity are complex, and they give several alternative explanations.
These potential influences will be difficult to unravel, but a better understanding of them may help to plan interventions, especially if similar signals operate in humans with their varied and less distinct eating patterns.

Comments