
Cutaneous leishmaniasis (CL) remains one of the most neglected of the neglected tropical diseases or NTDs. CL is caused by infection with the protozoan parasite Leishmania which is spread by the bite of an infected sand fly and resides inside human macrophages.
The disease results in disfiguring skin lesions and is strongly associated with extreme poverty, instability and conflict, with large outbreaks over the last few years in refugee camps across the Middle East. Symptoms may contribute or lead to stigma, mental health conditions and social exclusion which reinforces poverty in affected individuals. There are over a million new cases reported annually but this is likely to be a huge underestimation as formal reporting is not mandatory in many endemic regions such as Ethiopia.
Treatment options
Treatment of CL is very challenging. The most common treatment consists of multiple daily injections with a drug based on the heavy metal antimony. This is problematic from several different angles: the drug is highly toxic and difficult to administer, people may have to travel large distances to specialist clinics for injections and treatment failure is increasing. For a condition that is debilitating but not usually fatal, these combined factors make the option of formal medical treatment unfeasible for many of those affected.
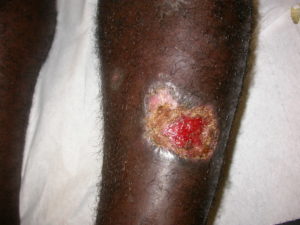
An alternative approach for treating CL is to exploit the sensitivity of Leishmania parasites to changes in heat, either with cryotherapy (e.g. freezing with liquid nitrogen) or thermotherapy. A major advantage of these physical methods for parasite killing is that they should work on drug-resistant parasite strains. Thermotherapy devices have been developed which heat the surface of the lesion to 50°C, with cure rates reported to be similar to those for the antimonial drugs. However, thermotherapy is very painful, requires administration of local anaesthetics and may result in second degree burns. The problem here is that the heat is being applied to the top of the lesion and needs to penetrate the layers of skin to the parasite-infected cells. Would it be possible to generate heat inside the infected cells instead?
A new approach
In our recent paper, we describe a novel method for killing Leishmania in the lab using a technique called magnetic hyperthermia, which is also being developed as a novel cancer therapy. This process involves the use of tiny iron-based particles known as magnetic nanoparticles. In the presence of an alternating magnetic field, these nanoparticles become magnetised and then relax, releasing localised heat into the cellular environment.
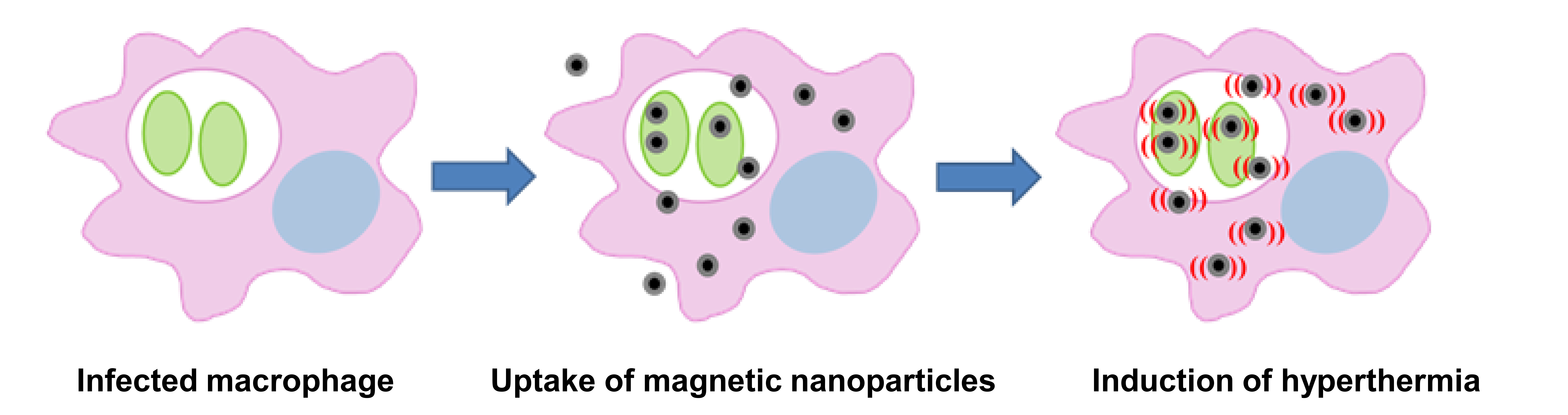
Our results showed that a combination of magnetic nanoparticles and an alternating magnetic field is very effective at killing in vitro cultured parasites, with changes in morphology similar to those killed by conventional heat treatment. We know that macrophages, as natural scavengers, take up these nanoparticles with high efficiency. We also have preliminary data (unpublished) that show that parasites inside the macrophage can be killed by this method with little effect on host cell viability.
The next stages will be to confirm these findings and to test the efficacy of this approach in an animal infection model. We will need to test whether there is any associated toxicity and if/how the nanoparticles are removed from the host when the lesion heals. However, magnetic nanoparticles are already being used for other medical applications such as MRI contrast agents and our studies in vitro show very low direct cytotoxicity.
We hope that in the long term our findings may help to develop new ways to treat one of the most neglected of the NTDs and improve the quality of life for people living with this disfiguring and stigmatising disease.

Comments