African trypanosomes cause human sleeping sickness in sub Saharan Africa but probably their more critical impact is through the disease they cause in the livestock of farmers and smallholders. Here, the economic losses of disease and the cost of prophylactic drugs to control the impact of the disease contribute to poverty. Breaking the cycle of disease transmission by targeting the parasite vector, the tsetse fly, provides an important means of disease control.
The trypanosome parasite is famous for its ability to avoid elimination in the mammalian host through its capacity to change its surface proteins and so stay ahead of recognition by host antibodies. This allows the parasite to be sustained long-term in chronic infections and so increases the likelihood of the parasite being picked up by tsetse flies during their blood meal, ensuring continuation of the life cycle.
The parasite uses an elegant approach to ensure the continuation of its life cycle. Although the parasite proliferates rapidly in the blood and tissues of its mammal host, the parasite controls its own population growth to prevent overwhelming the host too quickly. To achieve this, it monitors its numbers and, when a certain parasite density is reached, switches to a form that does not replicate- the so called ‘stumpy form’.
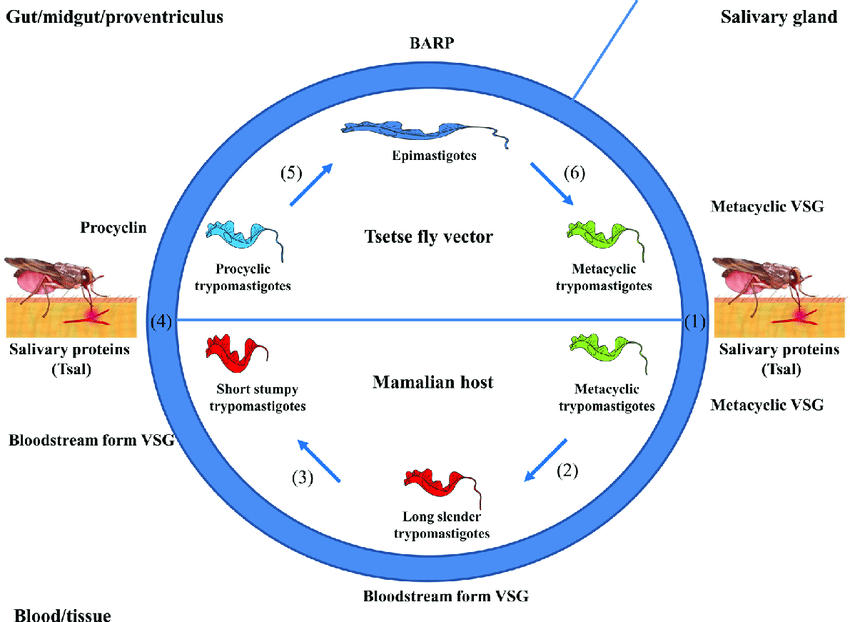
These forms are also adapted for survival when taken up by a tsetse fly in their blood meal. By generating these stumpy forms, the parasite both prolongs host survival and prepares for vector transmission – each increasing the chance of successful disease spread.
Quorum sensing
How the trypanosomes monitor their cell number in the mammal host – so called ‘quorum sensing’ – has long been a mystery. Similar behaviours are shown by many bacterial communities and these are often regulated by the release of small signalling molecules. Trypanosomes were thought to use a similar approach and biochemical assays had shown that the parasites generated a signal that was small (less than about 500 Dal) and stable in culture. The expectation was that this was a metabolite released and sensed by the parasites and used to assess their population density.
How does quorum sensing work?
Recent work by Rojas and colleagues has shed new light on how quorum sensing operates. They discovered that proliferating trypanosomes (‘slender forms’) express a surface protein, TbGPR89, that has the ability to transport oligopeptides- chains of a few amino acids generated by the breakdown of proteins. This surface TbGPR89 protein can drive the parasites to arrest their growth and develop into stumpy forms when over-expressed in the parasite, suggesting it may be linked to stumpy generation. Supporting this, the effect was not seen on parasites that had been selected to be unable to show quorum sensing through laboratory passage, or through the silencing of a gene previously shown to be needed for the sensing mechanism.
The ability of TbGPR89 to transport oligopeptides led to the exploration of whether oligopeptides could provide the stumpy formation signal to the parasites. This was found to be the case: by using complex mixtures of oligopeptides, Rojas demonstrated that trypanosomes in culture were effectively stimulated to become stumpy, and competent for further steps in the life cycle.
The role of peptidases
How could small oligopeptides be relevant in vivo? Work from several groups has shown that trypanosomes release digestive enzymes – peptidases – that break down proteins to oligopeptides in the bloodstream, and so Rojas and colleagues explored whether these could generate the stumpy forming signal. To do this, parasites were generated to express abnormally high levels of the peptidases. These were found to produce stumpy forms at lower than normal parasite levels and -importantly- the signal could also affect coinfecting trypanosomes where the digestive enzymes were not being highly expressed.
A new model
This has created a new model for trypanosome quorum sensing in which the parasites release enzymes that degrade host (and possibly parasite) proteins, and the digestion products- oligopeptides- are received by TbGPR89 to stimulate stumpy formation. This is very similar to mechanisms used by several bacterial species to respond to their environment.
Importantly, the identified mechanism is susceptible to new therapeutic approaches. The receptor molecule, TbGPR89, is essential for the parasite when it grows in the blood – parasites that lack the protein are not viable. This allows the search for drugs that block or inactivate this protein, which would kill the trypanosomes. Conversely, drugs that activate the protein – or mimic oligopeptides – can also prevent parasite growth by pushing the parasite to become stumpy too early- which might limit their virulence or prevent their transmission.

Comments