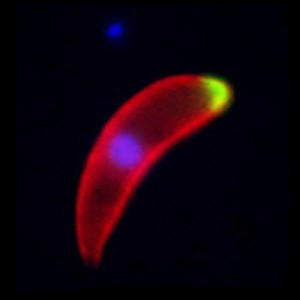
- A malaria ookinete ~ image from the Tewari lab
The most vulnerable stage of any parasite’s life cycle is generally the point at which it moves from one host to another. It may be exposed to a hostile environment and risk the chance of not being eaten by the next host or it may have to invade and establish itself in a different species, an intermediate host or vector. The malaria parasite is no exception. Once infected blood is imbibed by a mosquito any gametocytes that are present have approximately one day for fertilization to occur, and a motile zygote (the ookinete) to develop and escape from the rapidly deteriorating blood meal. Ookinetes must invade and traverse across the single-celled layer of midgut epithelial cells before they transform into a stage, known as the oocyst, which multiplies asexually to form sporozoites that invade the salivary glands. Any intervention that successfully blocked the ookinetes from entering these cells would inhibit the establishment of infection and break the transmission cycle.
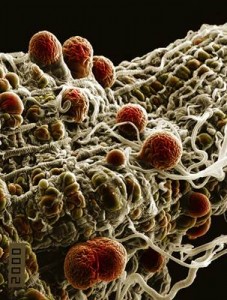
Oocysts on a mosquito midgut ~ image from the Hurd lab.
Until the turn of this century little was known of the molecular interactions that might occur between the ookinete and the mosquito midgut cells prior to invasion. By 2000 Zieler and Dvorak had established that ookinetes of two species of rodent malaria parasites penetrate the lateral apical surface of the cells; the invaded cells rapidly dying thereafter by a process that exhibits the hallmark of apoptosis. In the same year Carolina Barillas-Mury’s group produced further details of cell traversal and the fate of invaded midgut cells and proposed the “time bomb theory “, aptly named to illustrate the requirement for ookinetes to escape from these dying cells before they were shed into the midgut lumen.
Models exist that suggest the ookinete glides along the microvilli of the midgut cells and secretes proteins that cause transient binding prior to invasion. Several workers have speculated that, following this, invasion is initiated via one or more receptor / ligand interaction. This idea is supported by reports of several ookinete surface proteins that have been shown to be involved in the invasion process. Likewise, putative receptors on the surface of the midgut cells had been identified ,but interactions with ookinete surface molecules are not well characterised; the exception being a member of the P25 /P28 family of proteins on the surface of ookinetes of the human malaria Plasmodium vivax, Pvs25, which was shown to interact with calreticulin, present in the microvilli of the vector Anopheles albimanus. However, no functional studies were performed to show that this binding was involved in ookinete invasion.
Using a novel approach, Marcelo Jacobs-Lorena and colleagues originally identified a structural mimic of the ookinete surface protein enolase by screening a phage display library and named this peptide SM1. It was shown to inhibit the invasion of a rodent malaria and was hypothesises to interact with a mosquito midgut receptor and thereby block the natural receptor / ligand interaction.
The search for this midgut receptor has recently proved fruitful. Using a combination of midgut protein capture by double-derivatized SM1 molecules that would cross–link to the putative receptor and could be captured with streptavidin beads, and a subsequent ELISA, they showed that only one protein bound to SM1, namely, enolase-binding protein. Recombinant enolase from the human malaria parasite, Plasmodium falciparum, was shown to bind to this midgut molecule and the binding could be out competed with SM1. Using transgenic mosquitoes that secreted SM1, the group were able to select a population of rodent malaria parasites that were resistance to the blocking effect of SM1, even though enolase was still expressed on the ookinete surface. This and other data supports their hypothesis that invasion can occur by via different pathways. They confirmed that the previously identified requirement for the binding of plasminogen (from the blood meal) to enolase was necessary for both the SM1 susceptible and resistance parasite clones. Further experiments demonstrated that both a resistant clone of rodent malaria parasites and the human parasites was able to invade mosquitoes in which the enolase binding protein had been knocked down, thus demonstrating that a further receptor is involved.
The hunt for this additional receptor was now on. The group returned to their results from the original phage display library, where a second peptide with high binding affinity to the midgut lumen, named MP2, had been identified. Transmission blocking experiments using bacteria genetically transformed to express either SM1 or MP2 into the lumen of mosquito midguts were used to show that SM1 only caused significant inhibition of invasion in the susceptible rodent parasite clones, whereas the MP2 peptide inhibited both susceptible and resistant malaria clones. In addition the MP2 peptide significantly reduced the number of P. falciparum parasites that could invade the midgut and form oocysts.
The group propose that MP2 must bind to a universal mosquito midgut receptor. As there are five malaria species infecting humans alone, and these are transmitted by over 40 species of mosquito, this claim may be premature. However, the authors present a plausible model for midgut invasion and suggest that it is likely to be a multistep process, involving interactions between many parasite ligands and mosquito receptors, as is the case when the merozoite stage of the malaria parasite invades red blood cells. As they point out, the ability to recognise a variety of midgut receptors may be advantageous as it would enable particular parasite clones to infect mosquito species displaying different receptors.
This discovery has certainly moved us a stage closer to identifying a target for transmission blocking. Whether the MP2 receptor turns out to be the holy grail of this search, and blocking it would be effective against all human malaria parasites whichever mosquito species is transmitting them, remains to be seen.

Comments