Antimicrobial resistance (AMR) contributes to 4.95 million deaths per year, according to the WHO and, if nothing is done to curb the threat of AMR, this figure could reach 10 million deaths per year by 2050. Novel antimicrobials are needed, but there have been very few new antimicrobials developed, limiting the available treatment options against a growing list of AMR pathogens.
Carbapenem-resistant Acinetobacter baumannii (CRAB) are resistant to almost all antibiotics and are difficult to remove from healthcare environments. In fact, it is considered an ‘Urgent public health threat in US healthcare facilities’ by the US CDC. Polymyxins and some tetracycline derivatives are currently the only options for treatment, and even then the results are not consistent. There is a 40-60% mortality rate after CRAB infection. As a consequence, CRAB is classed as a ‘Priority 1: Critical’ pathogen by the WHO and is a top priority pathogen for new therapeutic development.
Thus, it is hugely significant that a new class of antibacterial chemicals have been identified as effective against them. Claudia Zampaloni and colleagues have identified tethered macrocyclic peptides (MCP) as a new class of antibiotic – the first new class to be identified in decades – and from within this new class of antibiotics zosurabalpin has been selected as the best clinical candidate.
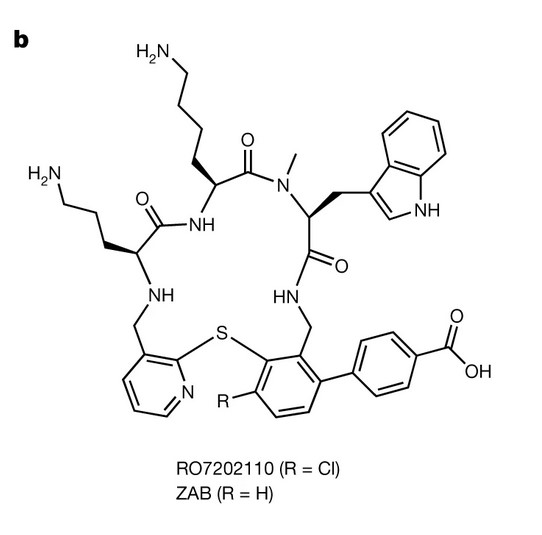
The outer membrane of Gram-negative bacteria, such as those belonging to the Acinetobacter genus, is made up of complex lipopolysaccharide (LPS) that act as a good protective barrier against antibiotics. To maintain this protective outer membrane, bacterial cells use a lipopolysaccharide transport system (made up of protein units) that are interspersed on the outer membrane. These systems transport LPS from the inner membrane to the outer membrane. Zosurabalpin targets the lipopolysaccharide (LPS) transport system in Acinetobacter, specifically the LptB2FGC complex, and thus blocks transport of LPS.
Zosurabalpin has been shown to be effective in vitro against 129 human clinical isolates of Acinetobacter baumannii, with a Minimal Inhibitory Concentration (MIC) of 1 mg l−1. This is far less than the MIC of the antibiotics currently being used to treat CRAB infection (tigecycline = 8 mg l−1 and colistin > 16 mg l−1). In vivo, the MIC of Zosurabalpin in mice is 2 mg l−1.
The publication of this and similar studies, gives much needed hope that new antibiotic classes can be discovered, and post-antibiotic, dystopian visions for the world remain only in fictional works.

Comments