An ancient, highly adaptable neglected tropical disease
Although the term schistosomiasis was coined in the mid 1800s–in reference to the morphology of the male adult worm, from the Greek ‘schistos’ and ‘soma’ meaning ‘split-body’–evidence of the disease causing blood flukes infecting humans dates more than 6,000 years ago.
The natural history of species of the genus Schistosoma nevertheless predates modern humans, and is marked by host-switching (once exclusive parasites of rodents, can now parasitise many vertebrate hosts), diversification (at present there are 23 different species of schistosomes), and geographical expansion (from its likely ‘out-of-Asia’ origins, it is now found not only in Asia, but also in Africa, the Middle East, South America, the Caribbean, and the Mediterranean).
Ongoing adaptation of this trematode is also emerging via hybridization. Viable eggs resulting from the sexual reproduction of blood flukes can be produced not only through pairings of the same species (for example, a female and a male Schistosoma haematobium couple), but also through the mixing of different but related species. Evidence of this latter process known as hybridization has been gathering across sub-Saharan Africa (see table 1 of Díaz et al, 2023), a region burdened by 90% of global schistosomiasis cases.
Remarkably, many of the reported hybrids are in fact mixes between a human schistosome (S. haematobium) and schistosomes of livestock (S. bovis, S. curassoni, S. mattheei). For example, since 2013 a S. haematobium x S. bovis hybrid strain likely imported from West Africa to Corsica, France has caused outbreaks of disease among people.
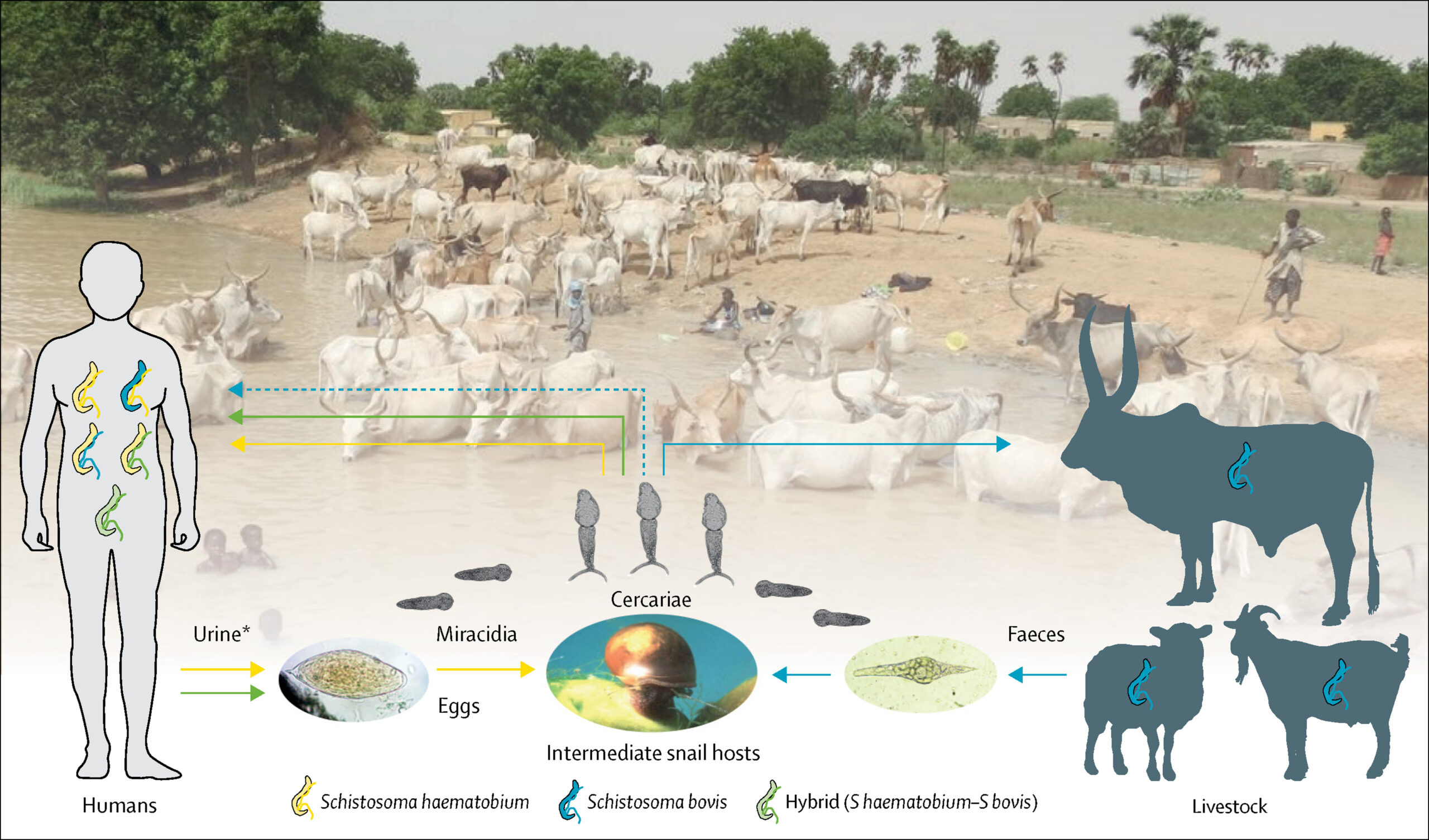
The recent detection of S. bovis in Nigerian children, as well as its discovery in the cattle of Zanzibar in a new geographical niche, further highlight a changing schistosomiasis picture.
The need for a One Health reframing
The high adaptability of the causative blood flukes generates unique challenges for the control of this neglected tropical disease, as well as for reaching the World Health Organisation (WHO) goal of its elimination as a public health problem by 2030.
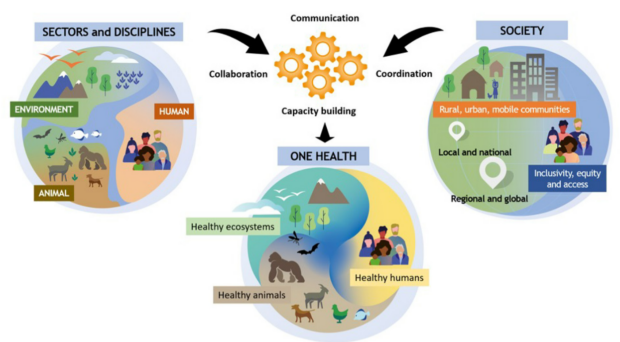
A recently published opinion piece in the Philosophical Transactions of the Royal Society B by Díaz and colleagues underlines the importance of a One Health perspective to reach WHO’s schistosomiasis elimination targets. A One Health viewpoint acknowledges the close connection between the health of people, the health of animals and our shared environment, and therefore, a departure from the traditional large-scale control interventions only focused on humans.
Measures like the treatment and/or vaccination of livestock, environmental management and control of the snail intermediate host, as well as improved sanitation, will have to be incorporated into existing programmes to reduce the chances of zoonotic spillover into humans and consequent reinfection. The prospect of elimination of this ancient water-borne disease requires work from a combination of disciplines at the human-animal-environment interface (for instance, epidemiology, molecular biology, computational modelling, human and veterinary medicine). Combating the threat posed by this multi-host, multi-species, infectious disease in a context of land use change, climate change, expanding trade and mobility of diverse populations calls for a new perspective.
Suggested further reading: ‘Spillover’ book by David Quammen.

Comments