
Dengue is a dangerous and debilitating vector-borne disease in many tropical and subtropical parts of the world, infecting 100 million people and killing 10,000 every year. It is caused by a group of phylogenetically related viruses, all transmitted by the tropical and sub-tropical mosquito Aedes aegypti. Given the peridomestic habits of this very adaptable mosquito, dengue is very difficult to control, despite the availability of many different control methods. While there is a commercially available vaccine against dengue approved by the FDA, it can only be used in people who already previous infections, otherwise there is a subsequent risk of severe dengue infection. Clearly, there is a need for better methods to control dengue and the mosquito that transmits it.
Wolbachia pipientis is an intracellular bacteria that naturally occurs and is common in insects. It has the ability to quickly spread and can be maintained in insect populations by transovarial transmission. The mechanism of cytoplasmic incompatibility, where infected males can only reproduce successfully with infected females, also increases the proportion of infected offspring in the next generation. There are many strains of Wolbachia, some specific to certain hosts. In 1980, Dr. Scott O’Neill, founder of the World Mosquito Program, started working on Wolbachia and dengue. His lab and others discovered in the early 2000s that Aedes aegypti mosquitoes infected with certain strains of Wolbachia are less able to transmit dengue virus and other viruses, such as chikungunya, Zika and yellow fever. Since this discovery, the World Mosquito Program, with the support of the Bill and Melinda Gates Foundation and others, conducted a number of laboratory and then fields trials, first in Cairns, Australia, followed by other parts of the world. The goal of these trials is to investigate and document the effectiveness of releasing Wolbachia-infected mosquitoes in reducing the burden of dengue (and other diseases) in the communities. Such trials have so far occurred in Vietnam, Brazil, Indonesia, Mexico, Sri Lanka, and many Pacific Island nations.
The World Mosquito Program and their local partners conducted a unique Randomized Controlled Trial of Wolbachia since 2017 in Indonesia, within the city of Yogyakarta. After obtaining community approval, they released Wolbachia-infected mosquitoes in 12 randomly chosen clusters across the city, while 12 other clusters were left untreated. Mosquitoes were deployed between 9 to 14 rounds in order to ensure adequate spread of Wolbachia in mosquito populations in the treatment cluster areas. Starting in 2018, residents who visited government-run primary care clinics in the area with fever and no other diagnostic symptoms were recruited to the clinical trial. Participants had their blood drawn and tested with a multiplex RT-PCR assay for arboviral infections. Participants with virologically confirmed dengue (VCD) infection were followed up to see if they were hospitalized or died.
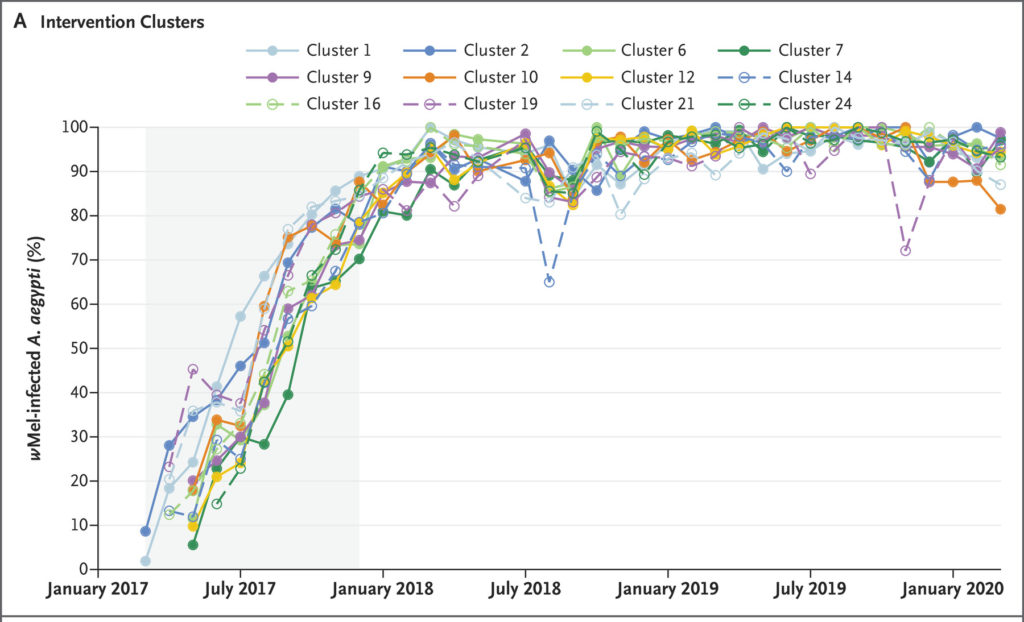
The wMel Wolbachia has quickly spread in the 12 treatment cluster areas, reaching close to 100% coverage by 2018, and even spreading into nearby control clusters to some degree over time. Dengue infections were 4 times less likely in people who lived in the treatment areas with Wolbachia-infected mosquitoes, compared to people living in control areas with regular mosquitoes. Wolbachia-infected mosquitoes were 77% effective in protecting people from dengue infection relatively to non-infected mosquitoes. Wolbachia infection in mosquitoes protected slightly better against serotype DENV-2 than against DENV-1. In addition, Wolbachia infection in mosquitoes was 86% effective in protecting against being hospitalized due to severe dengue. No participant died during the trial. The more people resided in areas with higher levels of Wolbachia infection in mosquitoes, the less likely they were to contract dengue, with about 80% of mosquitoes required to be infected for significant protection.
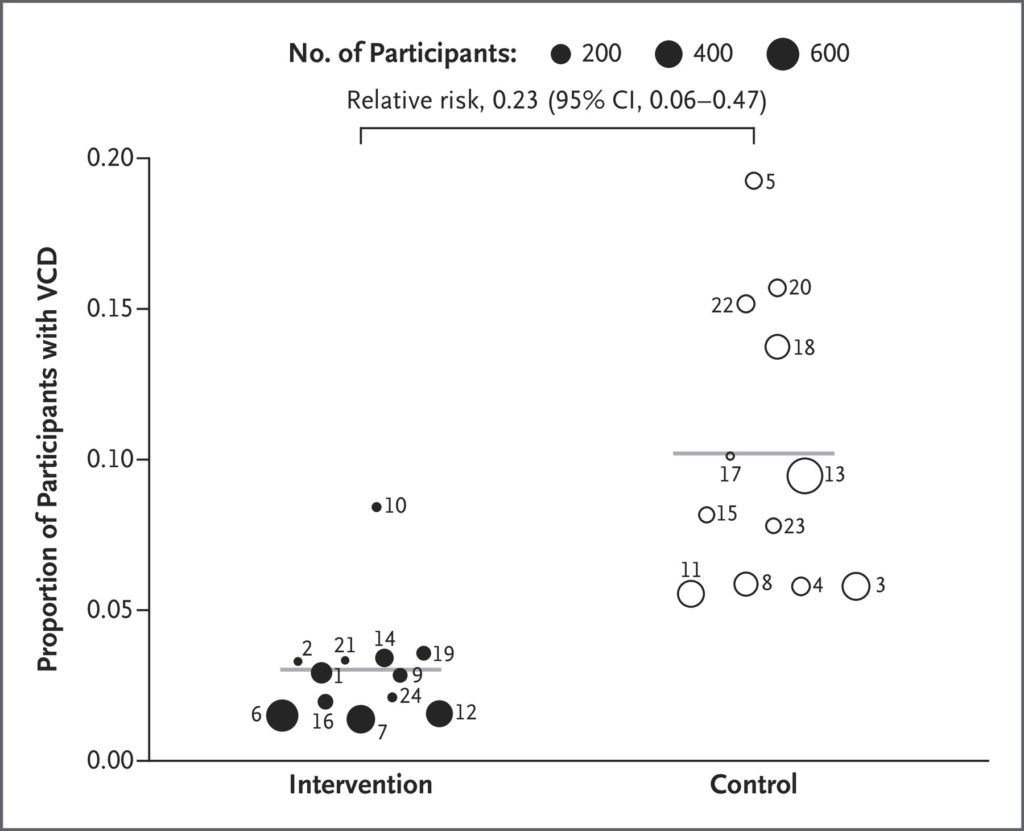
The results of this trial are certainly encouraging, showing an efficacy of these Wolbachia-infected mosquitoes on the same level as the efficacy of many COVID-19 vaccines in protecting us from SARS-CoV-2. While we don’t exactly know how and why Wolbachia-infected mosquitoes are less likely to transmit viruses such as dengue, proposed mechanisms include triggering innate immune effectors and changes to intracellular cholesterol transport. While dengue viruses could potentially develop resistance to this Wolbachia-induced blocking, such resistance has not yet been detected. In addition, it is possible for Wolbachia to disappear from infected mosquitoes. Therefore, there is a need to monitor mosquito populations for Wolbachia levels. Despite these facts, introgression of Wolbachia as a method to control arboviral transmission has clear advantages over other genetic control methods, such as the release of sterile insects, which require continuous or repeated releases to keep population levels down. This is not an issue with established Wolbachia-infected mosquito populations.
Introgression of Wolbachia into Aedes aegypti populations to reduce arboviral disease transmission is by now a well-established methodology, further demonstrated by the publication of this Randomized Controlled Trial. I’m looking forward to seeing the next steps in their endeavor to rid the planet of dengue (if not the mosquito) and other viruses. One day perhaps all Aedes aegypti on Earth will have Wolbachia in them, and no one will have to get sick or die from dengue ever again. Let’s work towards that!

Comments