
The introduction of an invasive species into a new habitat can result in considerable damage to ecosystems and biodiversity, the environment and the economy. Invasive species can have a negative impact on native species and their habitats through increased competition or predation, as well by introducing novel parasites and pathogens into their new environment, or acting as reservoirs for native parasites.
However, there may be an alternate scenario less considered, where invasive species act as a ‘sink’ for native parasites, thereby reducing parasite burden of native fauna. As parasites are taken up by a non-native host, the risk of infection is lowered for the native hosts as there are less parasites in the environment. In addition, the lack of co-evolution between the new host and parasite may prohibit the completion of the life cycle, thereby reducing the number of parasites released back into the environment.
There is accumulating evidence that infection of invasive species by native parasites is a widespread occurrence, however the ‘sink’ mechanism has as of yet only been investigated in a few invasive systems.
The cane frog: A rival or defender of native frogs?

A recent study chose to investigate this hypothesis using the invasive cane toad, Rhinella marina, in Australia. Cane toads and the native frogs of Australia both have their own distinct lungworm species; Rhabdias pseudosphaerocephala infects cane toads, while Rhabdias hylae is specific to native frog species. While the two genetically distinct parasites are adapted to their particular host type, experimental infections have shown that the two lungworm species can actually penetrate their non-native host. However, the parasite life cycle cannot be completed when the worm is not in its native host and the parasite dies.
In support of the ‘sink’ theory in this system, a field study found a lower infection prevalence of lungworms in native frogs in areas co-inhabited by cane toads, compared to the neighbouring areas without cane toads. This could potentially be explained by the cane toads acting as ‘sinks’ for R. hylae, thereby lowering the parasite density in the environment for subsequent infection of their native frog hosts.
Another possibility is that infection of native frogs by the cane toad lungworm may facilitate the frogs in developing an acquired immunity against subsequent infections by their own lungworm species. The adaptive immune system has previously been shown to have a role in enhancing the response of amphibians towards infections.
First come, first served
In order to test whether cane toads do indeed act as a ‘sink’ for native frog parasites, Felicity Nelson and colleagues report that they measured the rates of R. hylae larval uptake by 69 native frogs (30 Cyclorana australis and 39 Limnodynastes convexiusculus) and 31 cane toads. The experiment involved holding an anuran in a petri dish filled with 30 infective larvae for one hour, and then counting the number of larvae left following its removal. A second anuran species was then held in the same dish for an hour, and final number of larvae left were counted. These experiments were performed using different timing combinations for placement of frog species and cane toads, alternating each species as the first or second anuran in a petri dish.
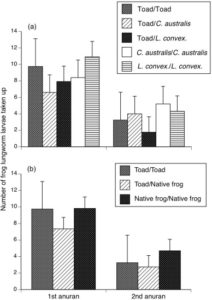
The experiment showed that the number of R. hylae larvae taken up by an anuran was reduced if it was the secondarily exposed species in the petri dish, regardless of whether the preceding species exposed was a frog or a toad. However, histological examination showed only 2 anurans had evidence of infection by larvae, potentially due to missed larvae in the other histology sections.
Infection protection, can nematodes boost immunity?
To identify whether infection by a non-native lungworm can ‘prime’ the host anurans immune system for subsequent infection by its native lungworm, the authors conducted a second experiment to determine whether prior exposure to R. hylae influenced the infection prevalence of R. pseudosphaerocephala in cane toads.
Seven cane toads that were pre-exposed to R. hylae larvae and seven control toads that had not been exposed were incubated with R. pseudosphaerocephala larvae. Cane toads were later euthanised for assessment of lungworm infection. No R. hylae larvae were identified in the cane toads 45 days after infection. However, few R. pseudosphaerocephala lungworms were found in toad lungs either following dissection after 20 days of infection.
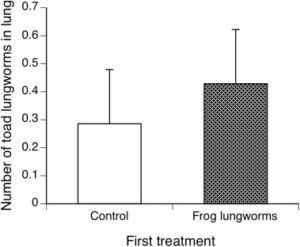
There was no significant difference in the uptake or number of the cane toad lungworms found between toads with or without prior R. hylae exposure.
Therefore, prior infection with the lungworm from native frogs (R. hylae) did not render a cane toad more or less resistant to infection by the cane toad’s own lungworm species (R. pseudosphaerocephala). While amphibians possess an adaptive immune system, which has been shown to reduce the intensity and impact of subsequent re-infections, this was found not to be the case for the cane toad and its native lungworm.
Summary
The current study implemented a simplistic experiment to assess the ‘sink’ mechanism in Australian cane toads for reducing the parasite numbers in native frog species. The current work demonstrated that invasive cane toads do have potential to act as ‘sinks’ for the lungworm of native frog species, and therefore areas that are co-endemic for both cane toads and native frogs may see a lower infection prevalence of the frog lungworm. However, the cane toads do not acquire immunity to their native lungworms from prior exposure to frog lungworms.
This research has presented encouraging preliminary data on the parasite-host dynamics and immunological responses in an invasive species system.

Comments