
On the 1st of February 2016, the WHO Emergency Committee declared the association between infection with Zika virus (ZIKV) and the rise in cases of congenital malformations and neurological complications a Public Health Emergency of International Concern. As noted therein, although a causal relationship was suspected, it was not yet scientifically proven. At that point the two outbreaks (ZIKV and microcephaly) were largely associated in spatial and temporal terms – an epidemiological association (covered by BugBitten in January). However, in recent weeks several studies have been described that provide convincing evidence that ZIKV infection during pregnancy may indeed cause catastrophic foetal abnormalities including microcephaly.
Initial evidence came from case studies
An expectant mother who had lived in Brazil in the early stages of pregnancy was believed to have been infected with ZIKV at the end of her first trimester. Ultrasonographic examination at week 29 showed the first sign of foetal abnormality. Following further complications and evidence of abnormality, the mother requested a termination that was subsequently approved. On delivery there was prominent microcephaly (defined as a head circumference at least two standard deviations smaller than the mean for sex, age and ethnicity) and autopsy revealed severe foetal brain injury. While there was no evidence of genetic abnormalities or other infections, particles consistent with ZIKV were detected in the foetal brain, along with higher levels of viral RNA than that reported from adult ZIKV-infected serum.
Birth abnormalities are convincingly linked to ZIKV infection
Recently a group led by Patricia Brasil investigated the link between ZIKV infection and congenital abnormalities by recruiting 88 pregnant women (at any week of gestation) who had recently experienced a rash. These women were followed up weekly with clinical and laboratory investigations, and with foetal ultrasonography at specific time points. Of the 88, 72 were positive for ZIKV in blood, urine or both. In 29 % of this cohort, ultrasonographic findings showed serious problems in foetal and central nervous system development. Importantly, there was no correlation between these abnormalities and the week of gestation that ZIKV infection had occurred, with infection as late as week 27 being associated with CNS abnormalities.
An important outcome from this study was the range of abnormalities detected. In addition to microcephaly, cerebral calcifications and intrauterine growth restriction (IUGR) were observed, in addition to two foetal deaths. This gives a death rate of 4.8 %; however, it must be emphasised that this cohort was selected on a symptomatic basis, which may equate to other important correlates e.g. a higher viral load. At the time of publication there had been only six live births, therefore outcomes remain unconfirmed. Importantly, one baby with abnormal ultrasonography was found to have normal measures at birth. It has been suggested that the reverse may also be found, with normal scans presenting with abnormalities at birth (Triunfol 2016).
Why is ZIKV so difficult to incriminate?
The criteria for incriminating an organism as the agent of a disease remain those determined by Robert Koch in 1890. The organism must be isolated from a symptomatic individual, used to infect a susceptible individual, and then re-isolated from the second individual. However, for obvious reasons these criteria are difficult to apply in the case of dangerous or rare diseases, therefore we often rely upon clinical and epidemiological evidence. In the case of ZIKV, the time delay between infection of the mother and diagnosis of foetal or neonate abnormalities provides one complication. Surveillance systems do not detect all ZIKV infections and the true number of pregnant women who were infected remains unknown – particularly those who had normal births. The situation is further complicated by a lack of reliable and rapid diagnostic tools. ZIKV can currently only be reliably diagnosed by polymerase chain reaction (PCR) of its RNA genome, and this test is only positive while the patient is viraemic (with an active viral infection). Serological assays, which detect antibodies to ZIKV (a flavivirus), are available but cross-react with other flaviviruses endemic in ZIKV affected areas, including Dengue. This means that although previous exposure to a flavivirus can be indicated, incrimination of ZIKV is difficult. When considering the symptoms, ZIKV in adults manifests in a similar way to Dengue and other viruses (when symptomatic), and in new-borns reports of microcephaly are considered ‘suspected’ until confirmation with MRI.
Retrospective analysis of the French Polynesia outbreak and ZIKV in model systems
Another key piece of evidence linking ZIKV infection with microcephaly resulted from the re-analysis of pre-existing data from the 2007 outbreak in French Polynesia. This was the largest documented outbreak prior to the current American epidemic, and no peak in microcephaly cases had been reported. However when four datasets were re-examined from the period, ZIKV infection occurring in the first trimester was associated with an estimated risk of microcephaly of about 1 %. This peak was likely missed as five of the eight affected pregnancies were terminated by medical abortion.
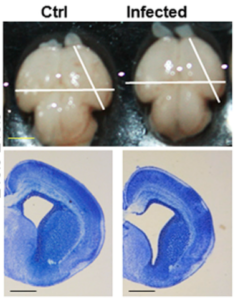
A number of recent publications focusing on ZIKV infection in animal models has provided a swathe of evidence which not only aids in establishing causality, but also begins to investigate the mechanisms by which ZIKV could cause foetal brain malformation. In one study two immunologically deficient mouse models were developed that support ZIKV infection (mice are naturally refractory due to their interferon profile). By using two different methods of interferon blocking, two mouse lines were generated that supported infection of varying severity. Pregnancies in mice with more severe infection resulted in foetal demise, while pregnancies in mice with a less severe infection profile resulted in viral infection of the foetal head and mild IUGR.
In two other studies, ZIKV-injected mouse foetal brains were found to be smaller five days later with corresponding effects on neuronal progenitor cell development, and the infection of pregnant mice with ZIKV resulted in microcephaly-associated signs including reduced cell number and cortical layer thickness. In the latter study infections were sustained by using mice that were naturally immunocompromised, and injecting a very high viral dose.
Finally, in a recent study human neural stem cells were cultured and subsequently exposed to ZIKV, and in another experiment the growth of ZIKV-infected brain organoids was observed. This resulted in the detection of a range of morphological abnormalities, including impaired cell growth and induced cell death, all of which corroborate neuronal cell death in ZIKV-infected foetuses.
ZIKV trajectory
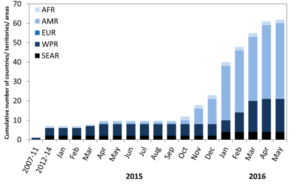
The number of cases of ZIKV in Brazil continues to rise, and 28 countries in the Americas have now reported the virus. Colombia has experienced the second greatest number of reported ZIKV cases after Brazil, yet because the outbreak there began in September, infected mothers will not yet have given birth. Here, the first ZIKV-linked birth defects were reported in March, with health professionals predicting a rise over the next 2-3 months.
Given the rapid spread of ZIKV, and fast-accruing evidence of an association with serious birth defects, all resources must be mobilised to allow an effective and successful response to this epidemic. As such the recent substantial pledge of ZIKV research funding by the U.S. senate is a welcome development.

One Comment