
This year, the Cancer Immunotherapy Keystone Symposia, was organized by four female scientific organizers of outstanding relevance in this field. Padmanee Sharma who uses a reverse translational approach to understand the resistance mechanisms to immunotherapy; Aviv Regev who investigates the biology of non- respondent patients by using a single cell approach; Crystal L. Mackall whose works focuses on enhancing the efficacy of CAR-T cells therapies and Kristen Hege who works in a range of novel therapies such as CAR-T cells, cancer vaccines and oncolytic viruses.
While immunotherapy has truly revolutionized cancer treatment and achieved clinical benefit for a subset of patients; much work needs to be done to increase the number of patients that will benefit from these treatments. Hence, this conference brought together leading and emerging scientists to discuss immunotherapy as a cancer treatment. Hot topics in this exceptional symposium included response and resistance mechanisms to cancer immunotherapy, technological advances for dissecting immune responses, engineered T cells and strategies for combinatorial therapies.
Approaches to improve the clinical benefit of immunotherapy
Immunotherapy is a biological therapy that helps the immune system fight cancer. There are several types of immunotherapies that can or either help the immune system fight cancer directly or enhance one’s own immune system response to attack these cancer cells.
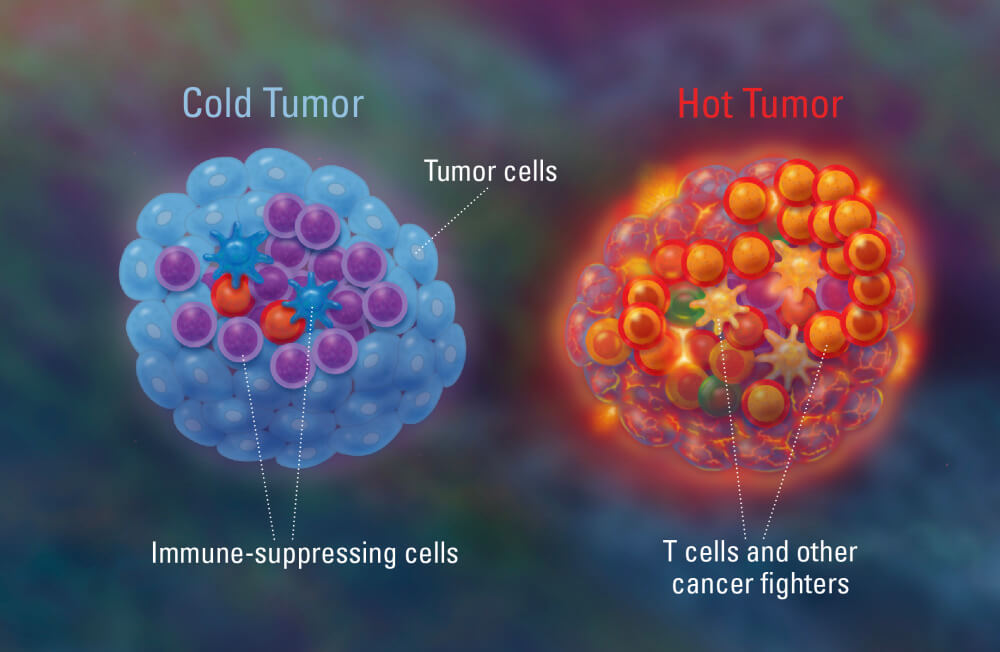
James P. Allison, a Nobel laureate and in attendance, paved checkpoint inhibitor immunotherapy by showing that blockade of CTLA-4 enhances T cell responses. However, the clinical benefit of checkpoint inhibitor immunotherapy is around 20-30%. This lack of clinical benefit can be, in part, explained by the so-called term “tumor resistance”. It is now well understood that tumors with high number of T-cells, “hot tumors”, have a better response to immunotherapy; whereas “cold tumors” do not respond well. Much research is now focused on the understanding of this mechanism as well as how to overcome tumor resistance.
One approach, discussed at the meeting, would be the identification of novel immunotherapy targets. Chris Takimoto presented data suggesting that inhibition of CD47, either alone or in combination, might enhance phagocytosis of cancer cells by macrophages. Similarly, Martina Seiffert elegantly showed that IL10/STAT3 signaling might be a novel predictive marker and a potential therapeutic target.
Combinatorial immunotherapy strategies are increasingly investigated. This approach was discussed by several key opinion leaders within the field such as Sandra Demaria and Antoni Ribas. With the aim to convert a “cold tumor” into a “hot tumor”, Demaria proposed the combination of radiotherapy with anti-CTLA-4 to induce immunity responses. On the contrary, Antoni Ribas, discussed novel drug combinations with anti-PD-1/PD-L1 in melanoma.
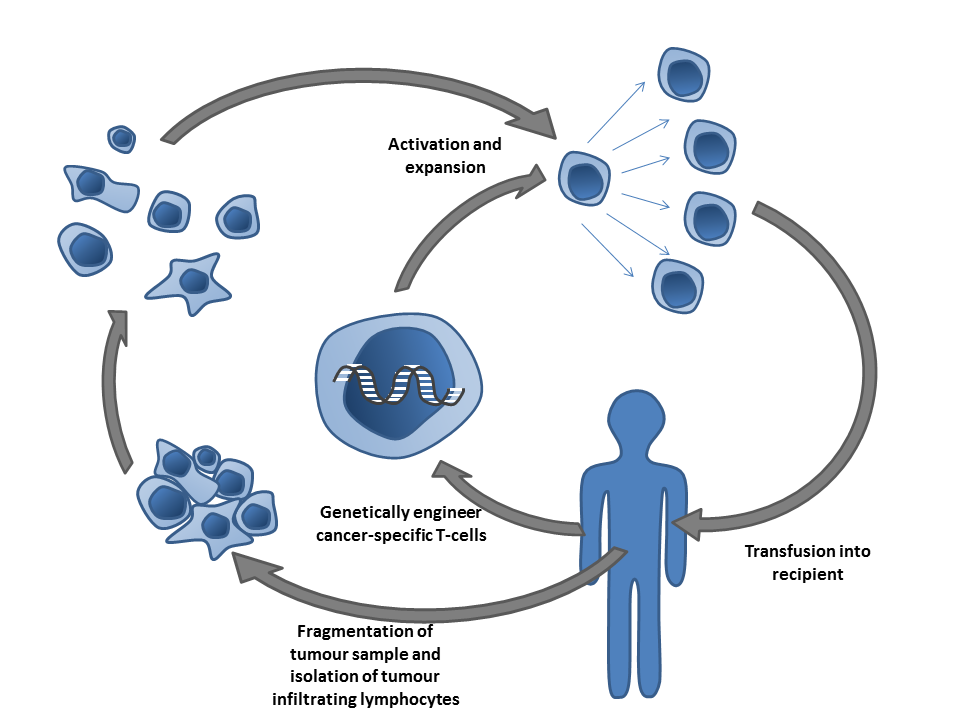
Another type of immunotherapy discussed extensively at the meeting was adoptative cell transfer (ACT), which aims at collecting and using patient’s own immune cells to treat their cancer. Advances using CAR-T cells, an ACT type that has developed faster in the clinic, were presented at the meeting. Carl H. June, a pioneer in the development of these cells, has lead several clinical trials. He presented the most recent advances and identified few barriers that need to be overcome in solid tumors. Evan William, a postdoc in Mackall’s lab, uses destabilizing domains to turn on and off CAR-T cells to defeat their exhaustion.
The future of cancer immunotherapy
The Keystone Symposia ended with a stellar session looking at the future of cancer therapy. Jennifer Wargo showed very nice data looking at the impact that intratumoral and the gastrointestinal microbiota play in the response to cancer therapy. She has beautifully shown that patients’ intratumoral bacteria can lead to chemotherapy resistance as well as negatively impact the anti-tumor immunity. She postulated big open questions to end this session. Can we modulate the microbiome to improve response to treatment? Can we use the “microbiome signature” as a biomarker for immunotherapy response? Current ongoing clinical trials might elucidate some of these fascinating questions.
Finally, Michael C. Jensen, took us a bit further in the future and explained how his lab uses synthetic biology to enhance CAR-T cells activity. He proposed a couple of synthetic solutions to overcome antigen scape as well antigenic heterogeneity by using synthetic phospholipid mimetic drugs as well as a CAR-T cell on and off synthetic approach to overcome cell exhaustion.
In summary, this has been a truly inspiring and diverse conference that has outlined the state-of-art in cancer therapy, has identified the most pressing answered questions and the areas that need more focus. The current efforts together with the development of new technologies as well as new approaches pledge a hopeful future for cancer patients.
BMC Medicine is part of a cross-sectional collection on Precision Oncology. It focuses on how precision oncology has shaped advances in the impact of non-coding RNAs in epigenetics and cancer, immunotherapy and tumor biology, and the clinical significance of various therapies in a range of cancers. This collection is open for submissions.
Comments