
This year, the Medical Dystrophy Association (MDA), combined, for the first time, a clinical and scientific conference with the aim to bring together world leading experts in neuromuscular diseases (NMDs). NMDs are a large, rare and a devastating group of diseases characterized by the impairment of peripheral nerves and/or muscles.
Many different topics were covered during the conference; some hot topics included gene therapies, practical implementation of new therapies, new results on currently undergoing clinical trials, DNA and RNA repeat diseases and newborn screening programs.
FDA and the new hope for neuromuscular disease
To date, treatments for NMDs have been, particularly challenging due to many shortcomings. Janet Woodcock, Director of the Center for Drug Evaluation and Research at the FDA, gave an overview on the current challenges facing NMDs. In particular, the difficulty to find patients for clinical trials, the lack of key clinical information and the slow progress of the disease, which might hamper the availability for funding, elucidates the need to develop new clinical approaches to study potential therapies in a timely manner.
Later in the session, an FDA panel discussed these challenges further. Among those, the ability to manufacturer gene therapy at a scale to bring optimal benefit to the public was raised by Peter Marks and discussed in depth by Nathalie Clements at the conference. Furthermore, strategies to facilitate the expansion of the study population pool of patients as well as the development of “N of 1” trial are needed.
In conclusion, in an era where many more gene therapies, cell therapies, antisense oligonucleotides (ASOS) and small molecules are being studied; new methods and trial designs must be explored.
Gene therapies
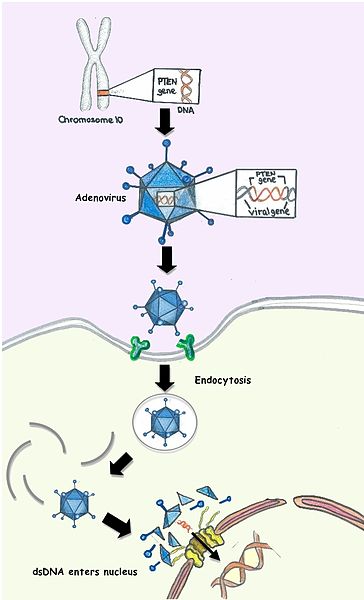
Gene therapy, a technique designed to introduce genetic material into a cell usually delivered by a vector, was discussed at the meeting. Adeno-associated virus (AAV), the most common vectors to deliver gene therapy, are modified so they do not cause disease and are able to deliver the DNA into the cell. Compiling data presented by Robert Brown, showed that gene silencing via AAV-delivered sRNA and microRNA is feasible for at least two ALS genes. Additionally, preclinical gene therapy using allele-specific knockdown with AAV9 is feasible to treat Charcot-Marie-tooth disease presented by Robert Burgess.
CRISPR-Cas9, a newly developed technique for gene editing, offers new possibilities for the treatment of NMDs. Among some of the advances presented at the meeting, Gene Yeo discussed the correction of DM1 by RNA-targeting
CRISPR gene therapy. Moreover, Melissa Spencer proposed a new CRISPR-Cas9 platform for the treatment of Duchenne Muscular Dystrophy (DMD) with a direct method of delivery using nanoparticles.
These and many other approaches were discussed at the sessions and further emphasized the current excitement in gene therapy and, in particular, for the treatment of NMDs.
Clinical trials
Clinical trials are the classical form to test whether successful preclinical candidates can be used to treat patients. While these have been implemented in other areas of medicine and have successfully defined new therapies, clinical trials for NMDs are just at the inception. A plenary session at the conference, discussed recent clinical trials‘ results as well as novel approaches and designs.
For instance, Jeffrey Statland presented results from the ACE-083 for Facioscapulohumeral Muscular Dystrophy (FSHD) study, a phase II trial currently underway. This locally acting muscle therapeutic showed no serious adverse events and it was tolerated in patients treated for up to 3 months. Phase 2 results from this trial will be released later in the summer.
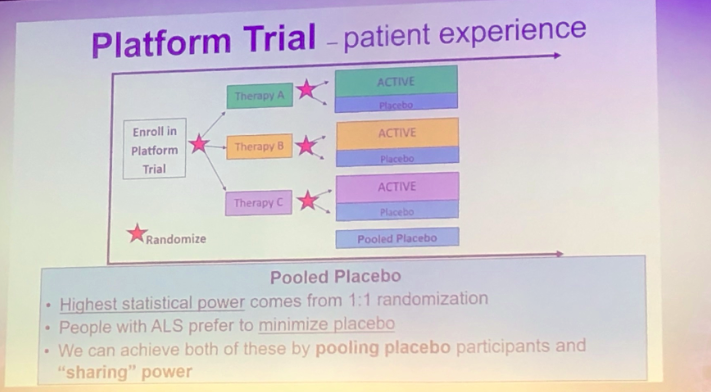
Interestingly, Sabrina Paganoni discussed a new trial initiative for ALS. Platform trials allow testing different treatments in a cost effective manner which provides efficiency of the process and creates an optimized trial infrastructure to study multiple therapies. These and many other trials for NMDs are currently underway which offer great hope for these diseases and will elucidate new treatments for these patients.
Newborn screening
In the 1960s, the concept of newborn screening (NBS) was coined thanks to the works of Dr. Robert Guthrie. He developed an easy and fast screening test to detect phenylketonuria, a developmental disorder, in newborn babies. Rod Howell, in attendance at the conference, presented an overview of the early days of newborn screening. While in 2001, 29 conditions were included in the newborn screening panel in the US; in 2018, 35 conditions comprised this panel including Pompe and Spinal Muscula Atrophy (SMA).
Pompe disease, discussed by Barry Byrne, was the first NMD accepted as a newborn screening disease in 2015. While Pompe disease has many genetic variants (500 genetic variants identified so far) and presents a varied disease spectrum, there are currently different therapy strategies available that made possible the addition of this disease to the NBS panel and its implementation.
Additionally, Richard Finkel discussed the current state for newborn screening of SMA, recommended in 2018 and currently under the pilot implementation period in US. SMA can be diagnosed performing a simple qPCR with high sensitivity and specificity and at a very low rate of false positives. Interestingly, other countries such as Spain and UK have implemented SMA as a NBS disease.
However, the case for DMD is different – presented by Peter Kyriacopoulos. DMD is not accepted as a NBS disease as no genetic screening is currently available due to the variability of the mutations. In addition, CK levels are difficult to interpret in newborns, genetic discrimination might occur and the perceptions of the child to be vulnerable or ill prior to the onset of any major symptoms are some of the risks for early diagnosis of DMD.
This extraordinary conference has combined leader experts from multiple disciplines to advance our knowledge and understanding of these devastating diseases. The combination of scientific and clinical sessions has provided an overview and the current state-of-the-art in our progress to treat and provide better quality of life for patients suffering NMDs.
BMC Medicine is seeking submissions for a new article collection: “New frontiers in diagnosis, treatment and management of neuromuscular disorder” guest edited by Dr Bradley Turner. We invite manuscripts and front matter content looking translational models of pathogenesis and therapy, clinical trials, diagnosis and screening programs and clinical care and management of NMDs.
Comments