
All of our food, one way or another, comes from crops, be they on our plate or in our cattle – and with nearly half of all under-five deaths being attributed to malnutrition, protecting crops from disease has high humanitarian priority – so it’s good news that a recent paper by Panagiotis Sarris and colleagues published in BMC Biology provides a promising resource for discovering new sources of disease resistance.
With nearly half of all under-five deaths being attributed to malnutrition, protecting crops from disease has high humanitarian priority.
Disease-causing microbes rely upon “effector proteins” that contribute to their virulence through manipulating nutrient delivery, or suppressing the host plant’s immune response. Hence, a possible route for protecting crops would be to genetically modify the targets for these effector proteins in such a way that they can still perform their function for the plant, but can’t be manipulated by effectors
Defeating Virulence
The viability in principle of such an approach has recently been demonstrated by Zhang and colleagues, in work on the receptor for jasmonate, an important plant signalling hormone. The jasmonate receptor is a common target of virulent factors, as its activation confers susceptibility to pathogens that complete their life cycle within a plant’s living tissue.
Pathogens such as Pseudomonas syringae, for example, are able to take advantage of the jasmonate receptor by releasing corantine, a jasmonate mimic. Guided by the crystal structure of the jasmonate receptor, Zhang and colleagues modified the receptor’s binding site in such a way that it can still bind jasmonate (retaining the physiological function), but can’t bind corantine – and this was shown to suppress P. syringae virulence.
Unfortunately, pathogen-host relationships are generally highly specific, and identifying effectors and their respective targets for every plant disease would be a monumental task – particularly as some of the most egregious pathogens, such as the rust fungus, are obligate parasites, and can’t be cultured. Fortunately, however, the plant innate immune system has evolved to do exactly that, and thus provides a generous resource for identifying new targets to be used in engineering disease resistance.
Stealing targets from plants
The innate immune proteins responsible for this useful resource are the so-called Nod-like receptors (NLRs), which recognise specific pathogen effector proteins that then trigger defensive responses in the plant. These typically number in the 100s in any given plant immune system, and they manage their recognition and defence-signalling functions through three domains – an N-terminal signalling domain, a nucleotide binding domain, and a leucine-rich repeat domain.
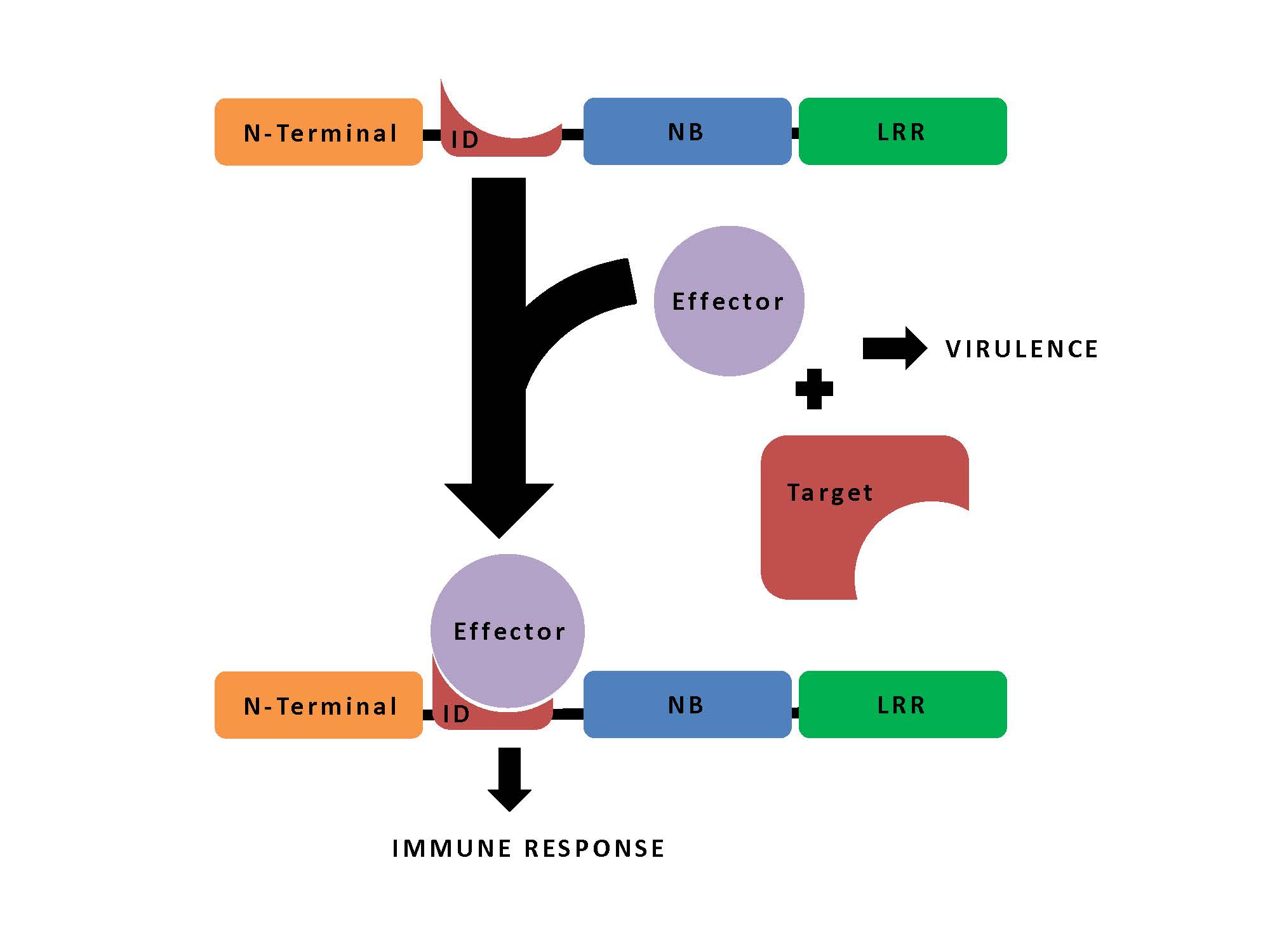
Some of them however have turned out also to contain “non-canonical domains” that they seem to have picked up from the targets of the pathogens’ effector proteins. Integration of the targets into NLRs thus enables them to act as “baits” to capture the effector proteins, which then trigger the defensive response.
In their study, Sarris and colleagues carried out an extensive search for possible bait sequences – known as NLR integrated domains (IDs) – in the genomes of 40 plants species, including important crops, such as maize, wheat and barley. From the identified 720 NLR-IDs, they report a unique 265 IDs; some of these hits overlap with known effector targets, or NLR-IDs identified in a previous screen, as would be expected; however, they also add many that are new.
Some of these targets, for example, may be more important to the pathogen than to the host – so their deletion could confer disease resistance.
A range of opportunities
Some of these targets, for example, may be more important to the pathogen than to the host – so their deletion could confer disease resistance. Alternatively, by showing the specific region of an effector target that is actually targeted, they can enable us to mutate the target in such a way that prevents interactions (as Zhang and colleagues did). More generally, they further our understanding of how NLR-fusions function, so that we may engineer our own from known effector targets
The realisation of these possibilities is still some way off; however, the present study by Sarris and colleagues, as well as a closely related study by Kroj and colleagues, brings us considerably closer.
More on the background to this research, and its implications, can be found in the accompanying commentary and research highlight, in BMC Biology and Genome Biology respectively.
- Mouse models, tau tangles and neurodegeneration in Alzheimer’s disease - 3rd January 2017
- Rooted in a changing environment - 23rd September 2016
- Learning from plants: new routes to engineering disease resistance - 22nd February 2016
Comments