
When COVID-19 first appeared on the scene, the scientific momentum to understand the disease demonstrated an unparalleled knowledge-building phenomenon. There is an irony to the fact that one of the newest diseases to humankind can be explained so thoroughly, but some ancient diseases still have major questions in need of answering. Fewer illnesses are more renowned for their devastation throughout history than the Black Plague – a bacterial infection by Yersinia pestis. The common model of transmission is schoolboy stuff – from rat, to flea, to human. However, this model is incomplete, and bacteriologists have remained stumped over the missing pieces required to explain the speed of the spread of Yersinia. Previously, we have discussed research showing how fleas can pass the bacteria to their young, adding an extra link to the network of transmission. A new study has gone further, demonstrating how a new vector, the body louse, could be a key component for explaining plague epidemics.
The concept of the human body louse (Pediculus humanus humanus) spreading the plague is not a new idea. We already know that these bugs, which can be found in clothes and bed linen in areas of poor hygiene, can spread other infectious diseases, including trench fever and typhus. Additionally, the epidemiology of plague epidemics does not make sense with only rats and fleas as we know them, the bacteria simply spread too slowly. Despite these facts, only a few investigators have attempted to test the infectious capabilities of the body louse. An outbreak in Morocco in 1945 gave a chance for an early study into this question. The colonial French doctors embarked to remove body lice from infected patients and then allowed them to feed on guinea pigs. Only one out of the ten animals become infected in this instance. Fast forward to 2006 and more French investigators, led by controversial professor Dider Raoult, decide to revisit the question with rabbits. For this they used a strain of body louse that had been long-adapted to feeding on rabbits instead of humans, which may have led to it losing features that facilitate plague transmission. Of eight rabbits exposed to highly-infected lice, only one did not succumb to the deadly condition. Although this rate of transmission was high, nearly all the lice themselves died only four days post-drinking an infected blood meal, which did not suggest that they would be proficient at passing on Yersinia bacteria (however, fleas themselves are damaged by the pathogens too).
Undeterred by the paradigm that body lice were poor vectors for Yersinia pestis, a team at the high-biosecurity Rocky Mountain Laboratories of the National Institute of Allergy and Infectious Disease in Montana, USA, decided to more thoroughly address the issue. Spearheaded by David Bland, in the lab of B. Joseph Hinnebusch, the team used a Pediculus lice strain that was more similar to those found on humans, and fed the bugs infectious bacteria through a membrane feeder.
Firstly, it was established that the insects were indeed infected by Y. pestis after feeding. Not only could they harbour the bacilli, but they could carry them for several days after exposure (the experiment did not carry on long enough to see exactly how long). The infected lice indeed fared worse than their healthy colleagues, however at most only 50% perished, showing that survival was better in a more realistic louse strain than that used on rabbits in the 2000s. Then, to ascertain how much bacteria could be passed on by the lice, they were given new sterile blood capsules to engorge on over the experimental time course, and the amount of Yersinia pestis bacilli in the post-feeding blood reservoir was measured. None of the pools recovered were lacking for the plague bacteria, all displaying at least some colony-forming unit activity (CFU – a measurement of bacterial quantity based on how many colonies form on agar plates). As lice can also transmit disease through their faeces, defecations were also collected and measured for CFUs, and too were found to contain pathogenic agent.
The investigators tested two scenarios of transmission; one in which lice could feed again immediately after being infected, and another in which lice were starved and kept at a lower temperature for 18h – to simulate being away from a host for a period of time. The latter group would aim to recreate what would happen to infected lice were left on clothes or bedding for nearly a day before they were used again. Although these lice had impaired survival, there was a signal for a slight increase in susceptibility to Y. pestis and even to ongoing transmission. This hints that the lice lose resistance to the bacteria when under stress (like when not being able to feed), or that the pathogen is adapted to surviving when the lice are not close to humans all of the time.
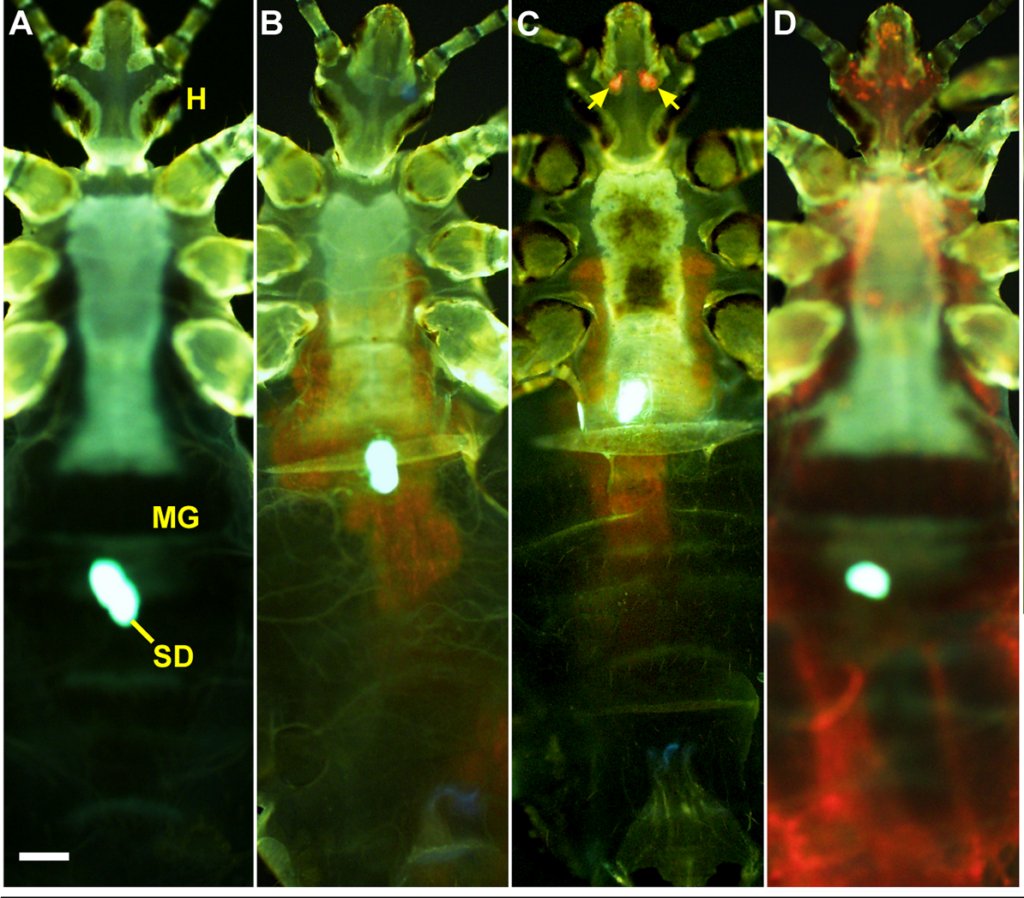
The research team next wanted to understand where the bacteria reside when they infect the lice. In fleas, the bacilli colonise the midgut, creating a biofilm that forces the insect to regurgitate bacterial colonies. The Yersinia strain used in this experiment was tagged with a fluorescent marker, so that it glowed red under the probing light of a specialised fluorescent microscope, allowing the researchers to find the bacteria inside the insects without even needing to dissect. The midguts of many of the bugs showed a clear red signal, however there were two other loci of infection observed in other individual lice; throughout the louse body (a critically high infection invariably lethal to the insect), or, more interestingly, two foci near the head. Where were these bacteria hiding?
The most obvious guess would be the salivary glands; the same site where malaria parasites await entry to a human host. However the shape of the fluorescence in the lice suggested otherwise; the oval objects of clustered bacilli did not match the anatomically u-shaped or bean-shaped salivary glands. There was, however, another set of glands near the head of lice; the Pawlowsky glands. These tiny organs have no known function (although they’re believed to lubricate the mouthparts), but they provided a perfect solution to the localisation puzzle. It also makes sense how the bacteria could get here with little trouble after a bloodfeed. Y. pestis could be carried on the proboscis and wash into the cavity of the glands, being deposited later (and after reproducing) via the lubricating mechanism.

So ambulatory lice could be infected in their Pawlowsky glands or their midgut, but can both these states transmit bacteria? Since the lice can be imaged while still alive, the experimenters separated them into midgut and Pawlowsky-infected groups and tested their ability to pass on Yersinia. As it turns out, the Pawlowsky gland-infected lice transmitted substantially more bacteria, demonstrating that this is the most potent mode of onward movement for the pathogen, and probably the most suitable target for blocking the spread of plague by body lice.
Humans and their lice have lived alongside one another for thousands of years, however this relationship is tinged with a deathly shadow. How much the spread of the plague bacteria by Pediculus humanus contributes to the harrowing epidemics remains to be established, but it is nonetheless humbling how a pathogen capable of civilisation-fold deaths still has many secrets being unveiled.

Comments