
Great progress has been made over the last 2 decades to reduce human malaria in Southeast Asia, with 80% fewer cases and 77% fewer deaths in the region compared to 2010 – the largest decline across all 6 WHO regions. All countries in the region have strategies in place to eliminate malaria by 2030, with some possibly realising elimination by 2025.
Despite progress, malaria remains a significant threat to public health across the region, and cases of zoonotic malaria are increasing, even in countries that are close to, or have reached elimination of human malaria (e.g., Singapore, Malaysia, and Brunei). The emergence of new zoonotic malarias is expected to complicate malaria control in the region.
Zoonotic malaria
The Plasmodium species responsible for most zoonotic malaria cases in humans is P. knowlesi, a parasite primarily of non-human primates (NHPs), including macaques. P. knowelsi has been identified in 10 Southeast Asian countries, and is now the dominant cause of malaria in humans in Malaysia.
As well as P. knowlesi, other simian Plasmodium species, such as P. cynomolgi, P. inui, and P. coatneyi, are increasingly shown to also infect humans throughout the region. Simian Plasmodium species are transmitted by Anopheles mosquitoes from the An. leucosphyris and An. dirus complexes, although evidence indicates that other highly prevalent mosquitoes, such as An. letifer, can do so as well.
Land-use change
These zoonotic malaria species were considered ‘forest malarias’ due to infections traditionally occurring in people working in the forestry industry. However, changes in land-use practises, including deforestation, mining, human resettlement, and expanding agriculture and urbanisation result in NHP habitat fragmentation, and are considered principal factors promoting the spillover of zoonotic simian malaria into humans.
These changes in land-use also increase the density of NHPs and mosquito vectors, and change their behaviours to promote interaction with humans (through seeking out food and blood-meals), resulting in increased contact between humans, NHPs, and mosquito vectors infected with zoonotic malaria. In their natural hosts, these NHP malarias are often chronic and mild/asymptomatic, making them ideal reservoir hosts for parasite spillover to humans.
Improving monitoring and surveillance in Indonesia
To date, understanding of zoonotic malaria, its mosquito vectors, and transmission dynamics is limited. It is therefore necessary to collect more epidemiological information, particularly regarding the zoonotic Plasmodium species circulating in NHPs, which species of NHPs are infected, and the mosquito species acting as vectors.
A recent paper by a group of researchers in Indonesia set out to do just this, by aiming to determine the prevalence and distribution of Plasmodium species in NHPs, mosquitos, and humans in five provinces in Indonesia – Central Java, North and West Sumatra, Aceh, and Central Kalimantan.
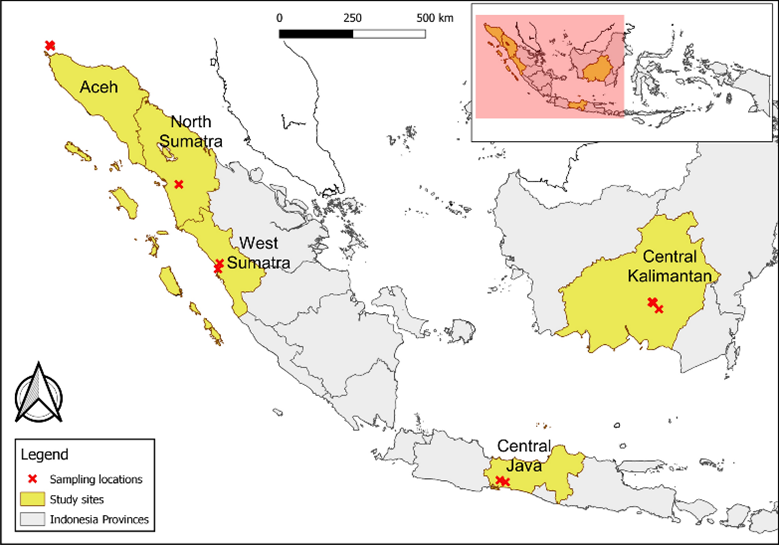
The research team trapped, anesthetised, and took blood samples from 110 macaques (Cercopithecidae) or Gibbons (Hylobatidae), which were subsequently released. 159 residents living near to the collection sites in Aceh and Central Java were also investigated for zoonotic malaria infections through blood spot microscopy.
Potential mosquito vectors were monitored in the five provinces using the human landing catch (HLC) method, and water bodies acting as potential breeding grounds were surveyed for mosquito larvae. HLC-collected mosquitoes were identified morphologically, and a subset were confirmed through molecular identification.
Prevalence of Plasmodium species in non-human primates and humans
Of the 159 residents that had their blood screened using microscopy techniques, no parasites were (luckily) identified. Of the 110 investigated NHPs, 47 (42.7%) had morphologically diagnosed Plasmodium infection, whereas molecular identification found slightly more (55) to be infected. Macaques had the highest infection prevalence.
At least 5 Plasmodium species were identified in the NHPs (P. inui, P. cynomolgi, P. coatneyi, P. knowlesi, and ‘other’ Plasmodium species), where P. inui (19) and P. cynomolgi (10) were most prevalent. The five provinces had similar NHP infection prevalence, although was particularly high in Aceh (Sabang).
Plasmoidum species identified in non-human primates from the five provinces. Permana et al (2023).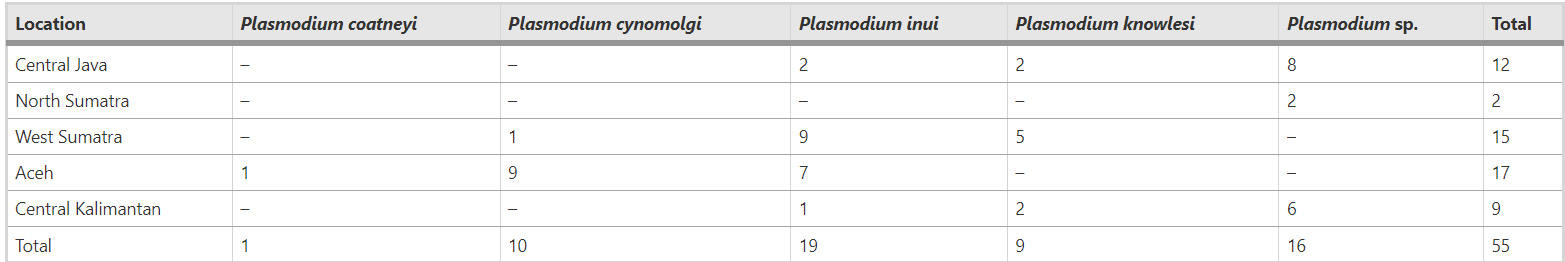
Identified mosquito vectors, breeding sites, and infection prevalence
Fourteen ‘types’ of breeding sites were identified, and included both natural (e.g., puddles, mangroves, and springs) and man-made sites (e.g., hoof prints, water containers, paddy fields, and wells).
Anopheles mosquito density was highest in Central Kalimantan, although were found in all provinces other than West Sumatra. Several Anopheles species were identified (e.g., An. kochi and An. dirus) but the predominant species collected was An. letifer, a known zoonotic malaria vector.
Only two adult An. letifer mosquitoes collected in Central Kalimantan were positive for Plasmodium DNA, although these weren’t identified to the species level.

Impact and implications
Although no humans or NHPs were positive for P. knowlesi in this study, humans infectioned with P. knowlesi have been previously reported in the Aceh region (Sabang) of Indonesia. This highlights the need for regular monitoring and surveillance in these regions, as well as for the pre-emptive implementation of efforts to control its spread.
Similarly, although An. dirus was not found here to be infected, it is a known vector across Southeast Asia. Anopheles letifer was, however, positive for zoonotic malaria, and thus confirmed as a zoonotic malaria vector in the in Central Kalimantan region of Indonesia. Both mosquito species likely transmit zoonotic malaria in the region, and should be monitored closely going forward.

Comments