
Schistosome species in Africa: blurring the lines!
Schistosomes affect over 200 million people worldwide. Their impact as a parasitic disease on human health is huge, second only to malaria. However, their impact on other animals, particularly livestock, are also potentially devastating. Cattle schistosomiasis is estimated to affect 165 million domestic cattle worldwide, potentially causing anaemia, weight loss, growth retardation, organ damage and in severe cases death.
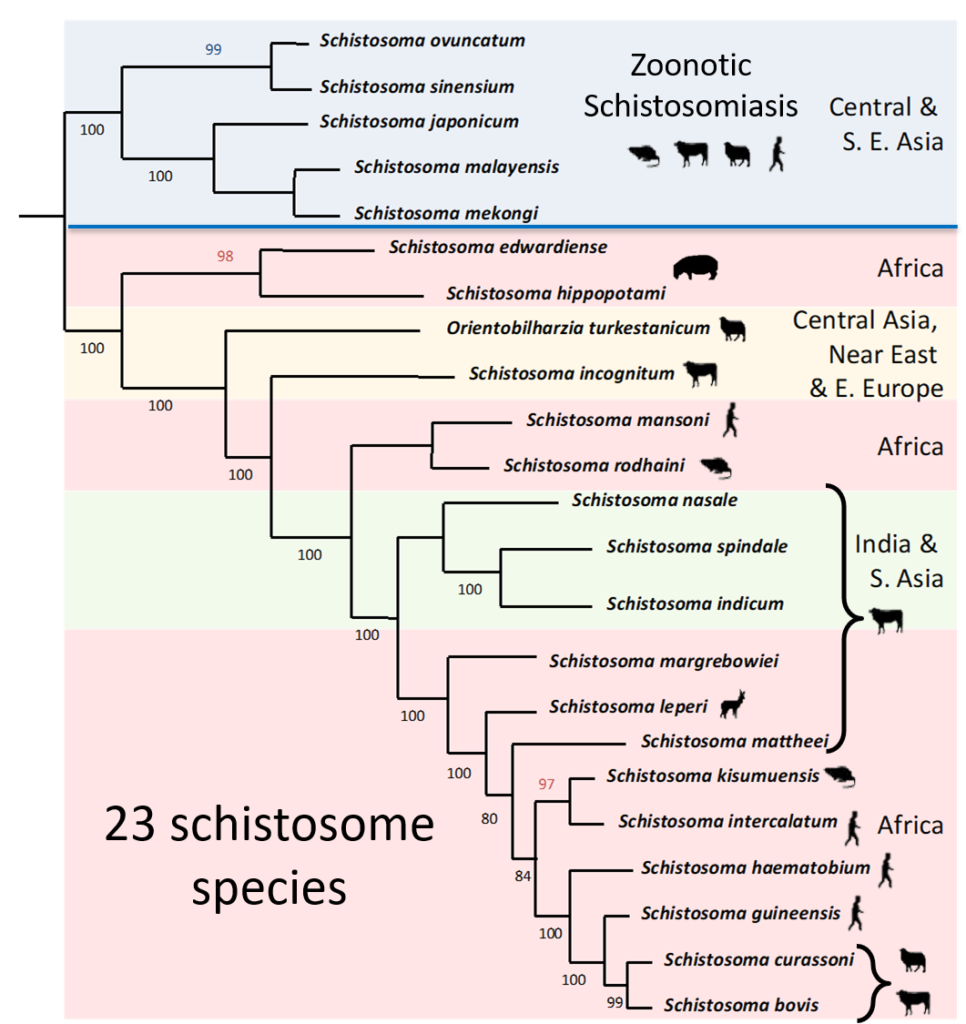
Unlike Schistosoma japonicum which is known to be zoonotic, it was traditionally believed that schistosome species in Africa were stricter with their definitive host species preference, particularly S. haematobium, considered to exclusively infect humans, and S. bovis, infecting only livestock. Furthermore, hybridization between different species of schistosomes, recorded in laboratory settings and occasionally reported in the field, was thought to be rare in nature due to the host preference of African schistosomes. However, recent findings using molecular techniques has thrown these assumptions in the air!
Where does hybridization occur?
Previous Bugbitten posts have highlighted the recent findings of hybrid schistosomes in human populations and there are projects underway to understand the geographical extent, epidemiological impact and genetic factors of schistosome hybrids and hybridization [Stothard et al 2020].
In a previous Bugbitten blog on the sex lives of schistosomes, Dr Bonnie Webster highlighted a key question: “Where are the adult worms of each species encountering each other?” S. bovis – S. haematobium hybrids have been found in human populations and in a rodent host in Senegal. However, until recently, there was little evidence on S. bovis in children, S. haematobium in cattle and S. bovis – S. haematobium hybrids in cattle.

Does schistosome hybridization occur in cattle?
In a recent study by Savassi et al 2020, researchers investigated whether cattle in Benin are infected by S. bovis or S. haematobium and whether introgressive hybridization occurs. [For useful definitions see here, to find out more about hybridization and introgressive hybridization and the concept of species see here].
To do this they collected stool samples from cattle and stool and urine samples from school children in a village in Benin. The stool samples from cattle and children were filtered and eggs collected. Some of the eggs were preserved for an egg morphology study, the others were hatched and a subset of the larval stages (miracidia) were used for molecular analysis. For the cattle specimens, a subset of miracidia were used to infect two different species of snail intermediate hosts. The authors then monitored the snails for cercarial (schistosome larvae) emergence, to record the chronobiology of cercarial emergence, an ecological parameter related to the behavior of the preferred definitive host:
- The cercariae of S. bovis, a cattle schistosome, emerge early in the morning, known as diurnal emergence.
- The human schistosome S. haematobium is known to emerge after midday, known as late diurnal emergence.
The authors preserved some of the emerging cercariae from each snail for molecular analysis.
Ecological and taxonomic findings
The molecular analysis confirmed previous findings that S. bovis – S. haematobium hybrids were present in humans in Benin. However, their research on the cattle samples were particularly interesting. The authors recorded the following 4 chronotypes based on the timing of cercarial emergence of the cattle-derived schistosomes from snails. The molecular analysis revealed interesting taxonomic groupings:
- Early diurnal emergence from snails, typical for S. bovis, and a molecular haplotype confirming S. bovis. This is the first finding that Benin has S. bovis in cows.
- Mid to late diurnal emergence from snails and molecular analysis showing atypical DNA profiles associated with S. bovis – S. haematobium hybrids. The authors highlight that this is the first evidence of S. bovis – S. haematobium hybrids in domestic bovines.
- A new chronotype of cercarial emergence for S. bovis, which is normally associated with early diurnal emergence, in this study the authors observed a second peak of nocturnal emergence around 7pm. The authors hypothesized that this could be linked to the behavior of cattle entering the water body to drink twice a day or could be linked to the water behavior of a nocturnal reservoir host the rodent. It could also be linked to hybridization between S. bovis and another species of schistosome. Molecular analysis grouped the cercariae that showed this chronotype in the S. bovis group.
- Another new chronotype shown by some cercariae was a late diurnal pattern, typical of S. haematobium, followed by a nocturnal peak at 7pm. Molecular analysis showed that this was another hybrid between S. haematobium and S. bovis. The authors call for more research into the link with these new cercarial emergence chronotypes and host behavior of cattle and rodents.
The egg morphology study revealed a high polymorphism in egg morphology from cattle and school children, with the greatest variation from cattle.
- Cattle: 60% of eggs from cows showed typical S. bovis egg morphology, 4% had typical S. haematobium morphotype and the remaining 36% showed varied intermediate morphotypes. The authors speculated that this is further evidence of S. bovis – S. haematobium introgressive hybridization occurring in cattle.
- Humans: No S. bovis morphotypes were found in the samples collected from children, 70% of eggs in children were of the S. haematobium morphotype, the remaining 30% were of an intermediate morphotype.
Main findings: this is the first evidence of S. bovis in Benin and the first evidence of introgressive hybridization between S. bovis and S. haematobium in cattle. This study also brought forward some very interesting information on the presence of differing chronobiological patterns of schistosome cercariae.
Zoonosis and One Health
This is the first evidence of S. haematobium and of S. bovis – S. haematobium hybrids in cattle. In the rural settings where schistosomiasis is prevalent, human and domestic animals live in close proximity. Cattle often roam freely in the areas surrounding villages and often use the same water sources. This is an ideal situation for schistosome infections and cross-species hybridization, potentially resulting in important consequences for public health measures to control and eliminate disease in humans and in cattle.
More studies have looked at hybridization in Senegal, Niger, Cote D’Ivoire, Malawi and others. Some findings seem to indicate that hybridization is not a common phenomenon while others indicate that these are frequent. Understanding the frequency, ecology and epidemiology is crucially important to understand where zoonotic transmission and reservoir populations in cattle could hamper human disease control and elimination efforts [Stothard et al 2020]. A One Health approach to public health that encompasses strategies and methods for the control, prevention and surveillance of schistosome infections in domestic animals would contribute not only to the well-being of the domestic animals we rely on, but would also strengthen the efforts to eliminate human schistosomiasis and support food and economic security and sustainability.

Comments