
In November, my team and I visited several villages in Senegal, collecting stool and urine samples from livestock, inspecting the blood vessels and offal of any slaughtered animal in the villages and surveying human and animal water-contact sites for specific aquatic snails. We were looking for schistosome parasites in order to determine the distribution and prevalence of the novel emerging zoonotic potential of these trematode worms in sub-Saharan Africa (SSA).
The research team, involving two new PhD students, Stefano Catalano and Anna Borlase, our Senegalese collaborators and myself, undertook this fieldwork in the region of Richard Toll of northern Senegal. This area is under important anthropogenic changes. Since the achievement of the Diama Dam in 1986 to develop agriculture, the prevalence of schistosomiasis dramatically increased in the region in both livestock and humans.

The current rehabilitation of the Lac de Guiers is also modifying water utilisation, bringing together animals and human in the same water areas and increasing the potential for animal and human schistosome species mixing.
Zoonotic potential of schistosome parasites
Schistosomiasis (or Bilharzia) is a serious Neglected Tropical Disease (NTD) of humans and animals, affecting poor rural communities in many parts of the developing world, with the greatest burden occurring in SSA. Schistosomes are transmitted through eggs passed in stool or urine, depending on the parasite species, which then infect snails in freshwater. The human or animal host becomes infected when they also enter water containing infected snails.
Environmental changes, through natural phenomena or human interventions, have a profound impact on the dynamics and distribution of schistosomiasis. They can increase the likelihood of human and animal schistosomes occurring in the same geographical area and in the same host type and thus have a dramatic effect on human and animal health.
Schistosomes in SSA have long been considered notoriously specific about the species of hosts they will infect. Historically it was thought that human schistosomes only infect humans and cattle schistosomes only infect cattle; remaining as distinct, conserved species. However initial studies, conducted on schistosomes obtained from both human definitive hosts and snail intermediate hosts in SSA, indicate that human and animal schistosomes can pair to produce novel zoonotic hybrids capable of infecting both humans and animals, influencing their potential for disease transmission and morbidity. Moreover these hybrid schistosomes appear to be occurring at extremely high prevalence and intensity levels in continued ‘hot spot’ transmission sites in SSA despite repeated mass drug administration (MDA) treatment programmes.
The implications of these findings are extremely worrying to human public health control programmes in schistosomiasis endemic countries. Little is known about the extent of the zoonotic transmission of schistosomiasis and its epidemiological implications.
The SHEEP (Schistosoma Hybrids: Epidemiology, Evolution and Prevention) project
Project SHEEP (Schistosoma Hybrids: Epidemiology, Evolution and Prevention) led by Professor Joanne Webster from the Royal Veterinary College, aims to elucidate, for the first time, the distribution and role of these novel zoonotic hybrid schistosomes particularly in regards to host range, drug efficacy, transmission potential and ultimately implications for successful and sustainable control. It is funded, for 3 years, under the new One Health ZELS (Zoonoses and Emerging Livestock Systems) research initiative between DFID and different UK research councils (BBSRC, ESRC, MRC, NERC an DSTL) and also involves co-investigators from Gaston Berger University in Saint-Louis (Senegal), from RISEAL in Niger and from the Natural History Museum.
The SHEEP project, implemented in 2 areas within Niger and Senegal in West Africa, focuses mainly on hybridisations within the S. haematobium group species, in particular hybrids between S. haematobium (human species) and S. bovis or S. curassoni (livestock species). However, we will also research the presence of these and other schistosome hybrids in the local wildlife.
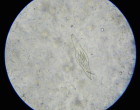
The presence of these hybrids has never been investigated in living livestock before; during our fieldwork we non-invasively collected stool and urine samples from hundreds of cattle, goat and sheep to look for and collect schistosome eggs.
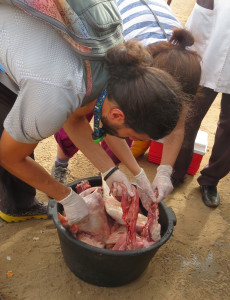
On slaughtered animals, we inspected the mesenteric blood vessels, the liver, the lungs, the intestines and the bladder in order to collect adult schistosome worms, trapped schistosome eggs and to examine the location of the parasites within their animal hosts. We also checked for the presence of other parasites of major veterinary importance, for example the liver fluke Fasciola gigantica. At human and animal water-contact sites we scooped for infected aquatic snails to test for and collect schistosome cercariae (the infective larval stage for the definitive host).
Back in the UK, we are now using new molecular techniques based on a multi-locus approach, analysing both mitochondrial and nuclear DNA simultaneously from individual specimens, to identify whether the species infecting the host is a human, animal or hybrid schistosome.
In January 2016 we will go back to these villages to collect urine and stool from school children and adults (in particular farmers, that go into the water with their animals) to determine the impact of hybrids in the human population.
Public and veterinary health impact of zoonotic schistosomiasis
This research will contribute to the major push to control and eliminate schistosomiasis as a public health problem, as recently put forward in the WHO and the London Declaration of the NTD coalition roadmap.

The findings will hopefully draw international attention to this recently discovered situation concerning the interactions of human and animal schistosomes. If the work confirms the existence of ‘hotspot’ foci of zoonotic infections and that novel zoonotic hybrid species are maintaining and exacerbating disease transmission, then new recommendations to public and veterinary policy will need to be made, including the implementation of concurrent methods for schistosomiasis control in livestock, akin to that currently employed for zoonotic S. japonicum transmission in China.
The SHEEP project will not only benefit people and their livestock in West Africa, but, by elucidating the interdependence between humans, animals, parasites and their environment, we hope that this research will enhance our understanding of a wide spectrum of multi-host parasitic diseases of humans and animals, and in particular the role of hybridisations within major taxonomic groups in our rapidly changing world.

Hi, i found your post very interesting and insightful. I’m currently ungoing my PhD research in Nigeria. Please I would like to seek your permission to make use of the image of S. bovis egg byStefano Catalano, for a publication I working on. I would also like to dialog further.Please do reply.
Dear Joy, thank you for your interest in this post. I apologise for the late reply, this post is by a guest author and so did not see this comment. If you still require the image I can try to find out if you can use it. Thanks for checking first! It is appreciated.