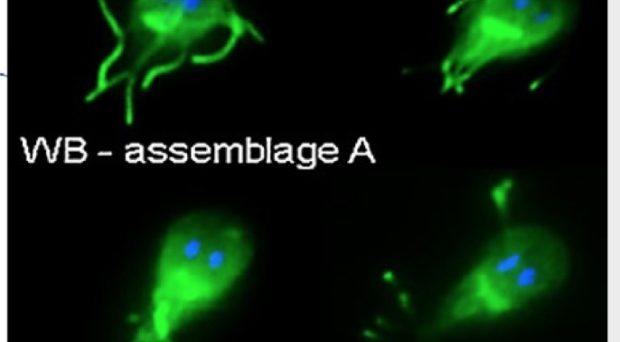
To win the fight against parasitic diahorreal disease, the molecular detail of pathogenesis must be understood. Three recent studies identified the Giardia-secreted proteins involved; highlighting the importance of virulence factors during infection and their potential as new drug targets.
Giardia and giardiasis
When Antony van Leeuwenhoek peered through his single lens microscope at his own diarrhoeal stool 300 years ago, he was greeted by a smiley faced swimmer, as he charmingly described:- “I have sometimes also seen tiny creatures moving very prettily; some of them a bit bigger, others a bit less, than a blood-globule but all of one and the same make. Their bodies were somewhat longer than broad, and their belly, which was flattish, furnished with sundry little paws, wherewith they made such a stir in the clear medium and among the globules, that you might even fancy you saw a woodlouse running up against a wall; and albeit they made a quick motion with their paws, yet for all that they made but slow progress.”
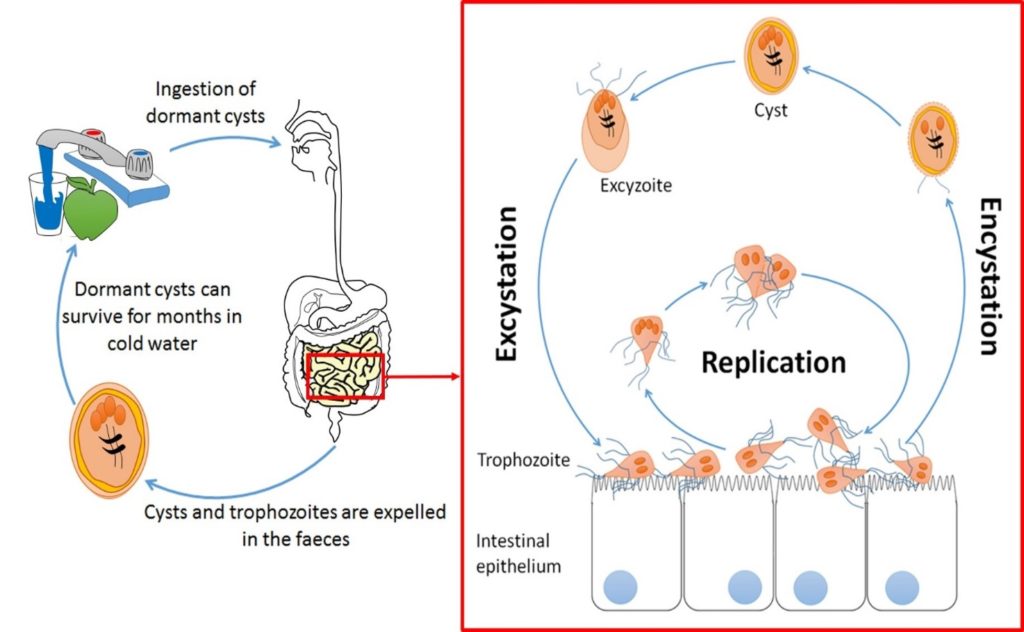
Giardia is transmitted via the ingestion of cysts from contaminated water or raw food and is responsible for over 280 million symptomatic cases of parasitic diarrhoea worldwide every year. After passing through the stomach, trophozoites emerge in the small intestine and colonise its upper part. Trophozoites are responsible for giardiasis, which can take the form of a watery diarrhoea – explosive and foul-smelling. However, clinical manifestations are highly variable ranging from acute to chronic infections with many, perhaps most infections remaining entirely asymptomatic. The established narrative of pathogenesis is trophozoite attachment to the duodenal epithelium via a ventral disc which sometimes (but not always) causes villus atrophication by apoptosis (programmed cell death) of surrounding cells, initiating inflammation and disrupting intestinal barrier function. With integral roles for parasite secreted intermediates such as proteases in the degradation of intracellular junctions having been described.
Secreted virulence factors contribute to Giardia pathogenesis
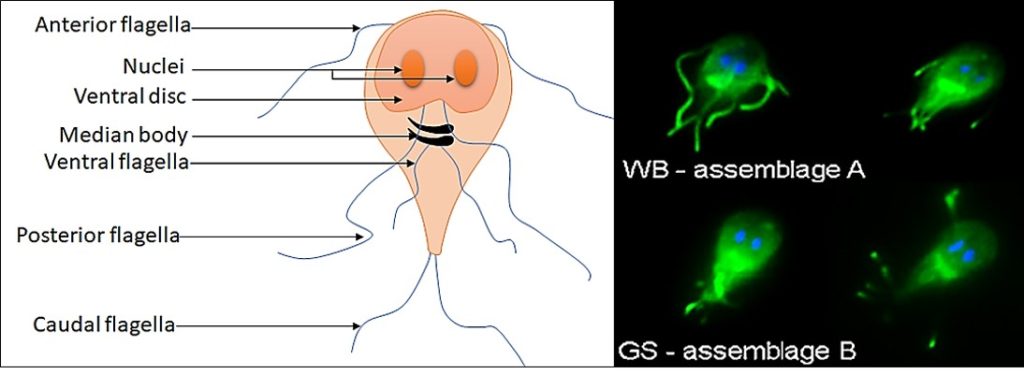
Giardia are single-celled eukaryotes diverging from the main eukaryotic tree close to its root and, consequently, divergent to ours in many aspects of cell biology. The endomembrane system of Giardia is peculiar in many respects, perhaps most notably the golgi apparatus is conspicuous by its absence. Considerable work has been done to investigate endocytosis. Two key sets of secretory vesicles have been identified and roles for the endoplasmic reticulum have been proposed. However, when it comes to steady state secretion by the trophozoite form little is known for certain.
Features such as the “bare patch”, an apparently vacuolar structure underlying the ventral disc, have been speculated to be a site for dynamic membrane trafficking akin to the trypanosome flagellar pocket. This might occur during host cell attachment and it has been proposed that upon attachment, upregulation and secretion of some virulence factors might occur and contribute to the localized damage and inflammation observed in the duodenum in some patients.
Localized damage cannot be the sole cause of the profound diarrhoea that can affect the intestinal absorption over a much wider area of the digestive tract than the site of colonization – suggesting a role for soluble, secreted, virulence factors.
The Secretome is a term used to denote all the secreted proteins in the extracellular space. These secreted proteins can be identified and quantified through mass spectrometry-based proteomics platforms. Three recent research articles published in Scientific Reports by Samantha Emery and colleagues, PLOS Neglected tropical diseases by Showgy Ma’yaeh and colleagues and in GigaScience by Audrey Dubourg and colleagues have trained the power delivered by proteomics and bioinformatics techniques on the questions of what Giardia trophozoites secrete, whether secreted proteins are Giardia lineage specific, how secretion is modulated by the environment and how the secreted products affect the intestinal epithelia.
Secretomes and infection
When Samantha Emery and colleagues addressed the secreted factors and mechanisms behind the origin of the infective cycle of attachment; they were able to show that trophozoites respond independently to host soluble signals early in pathogenesis, and that initial exposure to these secretions prompts a switch to a motile population phenotype. Conversely, Showgy Ma’ayeh and collaborators focused on identifying up-regulated secreted virulence factors from Giardia and evaluating their effects on the intestinal epithelia. They highlighted the importance of both host and parasite secreted proteins during the infection and the intricate and localized cellular responses occurring during a cognate interaction. Where the other two articles explored the effects of Giardia secretion upon interactions with the intestinal epithelia with which they were interacting, Audrey Dubourg and her colleagues produced a high resolution profile of the concentration of proteins being secreted by trophozoites grown in the absence of intestinal epithelia, to pinpoint the proteins secreted independently of colonisation or interaction with the host cells. They showed that Giardia trophozoites secrete only a very select group of proteins, but that these are secreted at quite high concentrations and are sufficient to disable the normal function of enteric epithelial cells even at very high dilution.
A new model of Giardia pathogenesis incorporating the secretome
Most of the proteins highlighted as secreted and up-regulated by trophozoites belong to three major group of proteins: Giardia tenascins, cathepsin B precursors (GCATBs) and high cysteine membrane proteins (HCMPs). Trophozoites also release other proteins with specific roles associated with niche adaptation.
From these studies, a novel mechanism of early pathogenesis has been proposed:
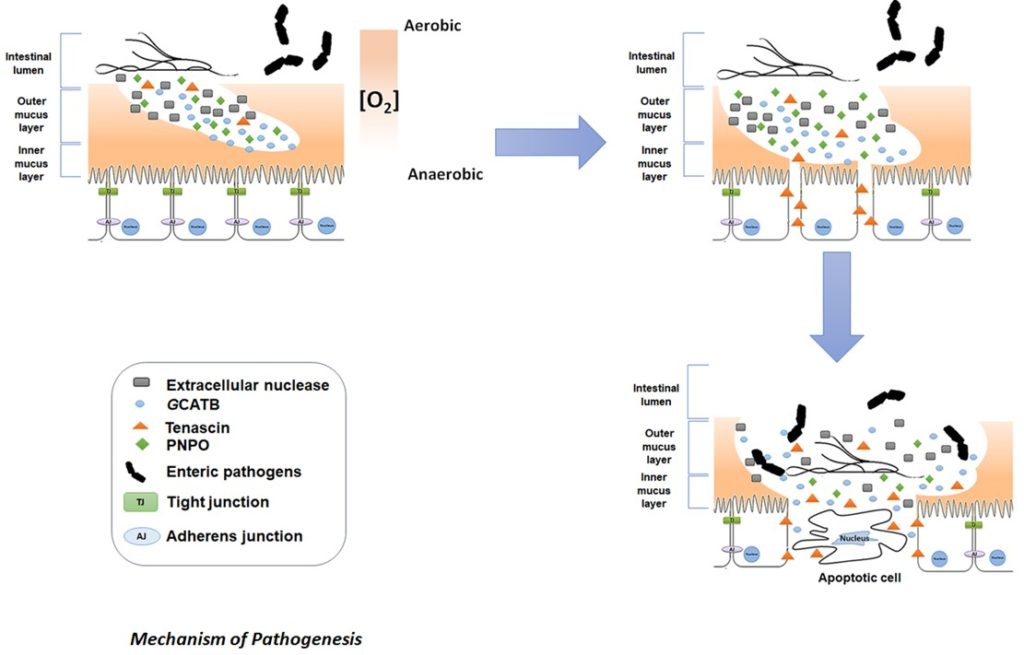
1. Pyridoxamine 5’phosphate oxidase (PNPO), secreted by Giardia, produces a reducing environment favouring the growth of Giardia trophozoites
2. Giardia extracellular nuclease degrades the outer layer of the intestinal mucus granting better access to the mucus layer to GCATBs
3. Further degradation of the protective intestinal mucus barrier may be caused by GCATBs as well as subsequent disruption of the intestinal intracellular junctions
4. Tenascins are likely to be responsible for reducing adhesion between epithelial cells and thereby maintaining intestinal cell separation. This is effected via ligation of epidermal growth factor (EGF) receptors present at the surface of the intestinal epithelial cells. This loss of epithelial integrity potentially driving increased apoptosis amongst more detached intestinal cells and at once dysregulating the absorption and barrier functions of the epithelium.
5. Giardia mediated dismantling of the barriers provided by the mucosal membranes and modulation of the environment leaves the intestinal epithelia prone to secondary infections by opportunist microbes residing in the intestinal lumen and sensitive to irritation by allergens in foodstuffs. Secondary infections likely contributing to further inflammation and to the characteristic symptoms of giardiasis.

Comments