
Every year, the Organization for the Study of Sex Differences (OSSD) and the Editor-in-Chief of Biology of Sex Differences select an outstanding paper published within that calendar year and present the author with the OSSD-BSD Publishing Prize during the annual meeting. This year’s winner was Dr Joanna Floros. This blog, authored by Dr Floros and her associates, outlines her beginnings as a sex differences researcher and the findings of her award-winning paper.
A sex differences researcher is born
After my postdoctoral training, I (Dr Joanna Floros) started working with a clinical group in neonatology in the department of Pediatrics at Harvard Medical School. The research focus was on pulmonary surfactant, a lipoprotein complex which is essential for life because it lowers surface tension and prevents the lungs from collapsing. Prematurely born infants who lack pulmonary surfactant or have insufficient amounts of surfactant have breathing problems and can develop Respiratory Distress Syndrome (RDS). Prematurely born male infants had a higher risk for RDS as surfactant production is a bit delayed. Hence from the very beginning of my career in pulmonary research, I realized that sex is an important variable to consider in my studies.
 To this day, pulmonary surfactant proteins remain the primary focus of my lab. These proteins (particularly the protein “SP-A,” which stands for surfactant protein A) along with the alveolar macrophage (“AM,” also known as dust cells for the role they play in ‘cleaning’ the lungs of potentially harmful particles), are the first line of defense against a number of harmful pathogens, allergens, and pollutants, such as ozone (O3). In humans the SP-A chromosomal locus consists of two functional genes, which encode the SP-A1 and SP-A2 proteins, respectively. Each gene was further identified with several SP-A-based variants that differ within the coding sequence. Genetic studies showed that these SP-A1 and SP-A2 variants associate with various pulmonary diseases, and in vitro studies showed functional, structural, and regulatory differences between the two gene products and among variants. At this time, it became imperative that we study these variants in vivo and this led to the creation of humanized transgenic (hTG) mice in which each mouse carried a different human SP-A variant and the gene encoding the SP-A variant is knocked out (KO).
To this day, pulmonary surfactant proteins remain the primary focus of my lab. These proteins (particularly the protein “SP-A,” which stands for surfactant protein A) along with the alveolar macrophage (“AM,” also known as dust cells for the role they play in ‘cleaning’ the lungs of potentially harmful particles), are the first line of defense against a number of harmful pathogens, allergens, and pollutants, such as ozone (O3). In humans the SP-A chromosomal locus consists of two functional genes, which encode the SP-A1 and SP-A2 proteins, respectively. Each gene was further identified with several SP-A-based variants that differ within the coding sequence. Genetic studies showed that these SP-A1 and SP-A2 variants associate with various pulmonary diseases, and in vitro studies showed functional, structural, and regulatory differences between the two gene products and among variants. At this time, it became imperative that we study these variants in vivo and this led to the creation of humanized transgenic (hTG) mice in which each mouse carried a different human SP-A variant and the gene encoding the SP-A variant is knocked out (KO).
2017 OSSD-BSD Publishing Award paper: a summary of the research
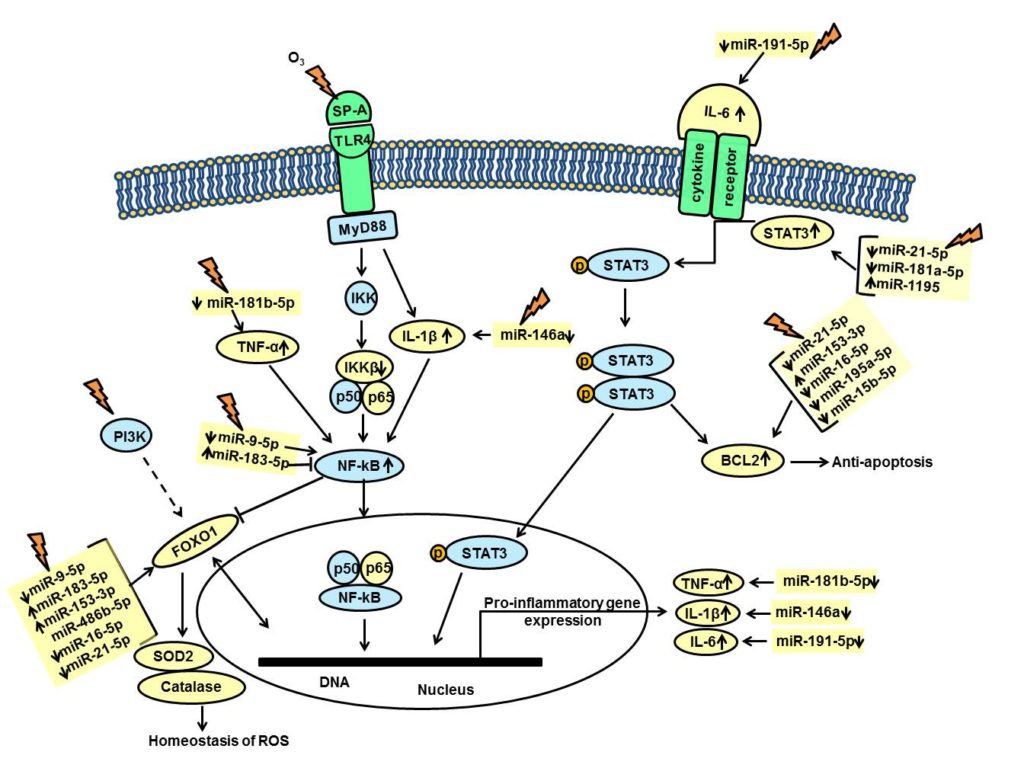
Environmental pollution (especially O3-induced oxidative stress) not only negatively affects lung function and inflammation, but differentially affects SP-A variants. We and others have shown that in the absence of SP-A, the negative effects of pneumonia and other types of lung injury increase. Moreover, sex differences have been observed in the survival rates between male and female wild type and SP-A KO mice after bacterial infection alone or preceded by O3-induced oxidative stress. Females exhibited a better survival rate in response to infection alone, but when oxidative stress preceded infection the survival outcome was reversed with males doing better. It was clear that sex hormones play a role in this outcome because the sex differences in survival of spayed/neutered mice were not observed with great frequency. Sex differences in the AM proteome as a function of the SP-A genotype have also been observed. Because SP-A plays key roles in innate immunity of the lung by modulating the removal of pathogens (including bacteria) via phagocytosis by the AM and inflammatory processes, both of which are central to several pulmonary diseases, in our study we sought to study mechanisms of the impact of O3-induced oxidative stress on the regulation of the AM miRNome as a function of SP-A genotype and sex.
We hypothesized that the two human SP-A1 and SP-A2 gene products differentially regulate the AM miRNome in male and female mice either in the presence or absence of oxidative stress. MicroRNAs (miRNAs) are short non-coding RNAs involved in post-transcriptional regulation of genes and play a key role in diverse biological processes. Numerous miRNAs have been identified in mammalian cells and up to one third of all protein coding genes is regulated by these small molecules.
We exposed male and female mice expressing SP-A1 or SP-A2 to O3 or filtered air (control) and measured the expression levels of 372 miRNAs. The miRNAs whose expression was significantly altered in response to oxidative stress were identified and used to identify pathways and molecules involved and potentially affected by these miRNAs. The findings showed that the AM miRNome is differentially regulated by the two SP-A genes in response to O3 exposure and that the SP-A2 male (but not female) miRNome is associated with genes involved in inflammation pathways, regulation of reactive oxygen species, and cell death (summarized in Figure 1), indicating that the pathways involved are sex-specific. The involvement of sex hormones in the SP-A2 sex-specific miRNome findings was supported by our findings in spayed and neutered mice where the regulation of the miRNome of the SP-A2 male mice compared to that of female mice in response to oxidative stress was significantly altered after castration. These findings support previous observations of sex differences in survival.
Even though both males and females have similar respiratory requirements, sex has been identified as one of the several risk factors for lung disease identified in humans. Sexual dimorphism in lung function can significantly influence the clinical outcome and modulate lung disease susceptibility, as is the case in respiratory infections, COPD, asthma, and CF, and in asthma exacerbations in response to O3 exposure.
The findings of the present study provide insight into mechanisms via which some of the sex differences may occur. This information in the future may contribute to considerations made for appropriate sex-specific treatment therapies and/or avoiding negative side effects that one sex versus the other may incur or even lack of efficacy altogether of a given drug or therapy in males versus females or vice versa. Moreover, regardless whether sex studies involve animals or humans, not all animals/humans have the same clinical outcome, indicating that other factors contribute. In the present study, we observed that the SP-A genetics play an important role in the outcome after O3-induced oxidative stress. This is important because in order to make advances into personalized drug therapies we need to take into consideration a number of factors that include sex, genetics, and environmental influences.
Dr Joanna Floros, Dr. George Noutsios & Dr. Nithyananda Thorenoor
Dr. George Noutsios was born and raised in Thessaloniki, Greece, has a PhD in Biochemistry/Molecular Biology from Aristotle University of Thessaloniki. He joined University of Arizona in 2016 where he was involved in a collaborate effort to unveil the primary mechanisms of chronic sinusitis in upper airway diseases. In 2018, he joined Arizona State University as Research Assistant Professor where he conducts his own independent research focusing on deciphering the role of surfactant protein A in the development of bacterial sinusitis utilizing ex vivo and in vivo air-liquid interface models of airway epithelia.
Dr. Nithyananda Thorenoor, a postdoctoral fellow in Dr. Joanna Floros lab at the Pennsylvania State University College of Medicine, and been involved in studies on alveolar miRNome and the impact the innate host defense molecules (i.e. surfactant protein A (SP-A)) have on alveolar epithelial cells and alveolar macrophages (AM). Dr. Thorenoor obtained his Ph.D. in Biomolecular Science from Korea Institute of Technology, Seoul, South Korea. He did his postdoc training at CEITEC, Masaryk University, Brno, Czech Republic, where he worked on the role of long non-coding RNA ZFAS1 in colorectal cancer cell proliferation and apoptosis before joining Dr. Floros group.
Comments