Malaria is a deadly global burden
Female Anopheles mosquitoes transmit the Plasmodium parasites that cause malaria. Control methods targeting mosquito populations, such as insecticide-treated bed nets, have greatly decreased the malaria burden in recent decades. Nevertheless, malaria remains a devastating cause of disease and mortality in tropical and sub-tropical regions, with over 400,000 deaths and 220 million cases in 2019. To progress further in the fight against malaria, we need a deeper understanding of the factors affecting transmission, as mosquito populations are becoming increasingly resistant to insecticides.
Plasmodium development in the mosquito is complex
A key determinant of malaria transmission is the time it takes for parasites to develop within mosquitoes. After a mosquito ingests malaria parasites in a blood meal by biting an infected person, the parasites progress through a series of developmental forms until they finally invade the mosquito salivary glands and then can be transmitted in a new bite. The time between infection of the mosquito and the ability for onward transmission is known as the extrinsic incubation period (EIP, about 10–14 days in Plasmodium falciparum laboratory infections). Its effects on transmission are linked to mosquito mortality: in mosquitoes with the same lifespan, parasites with a short EIP can be transmitted sooner and for a longer period of time than parasites with a long EIP.
The EIP is known to be affected by environmental temperature, as many growth processes are, but it is also linked to available nutrient resources within the mosquito. Our previous work showed that a reduction in the number of eggs a female produces increased parasite size and shortened the EIP, accelerating the appearance of parasites within the salivary glands. Parasites were effectively able to exploit the accumulated lipid resources available following egg development.
Mosquitoes naturally feed multiple times
Given the link between EIP and resources in the mosquito, our latest study considered the impact of a mosquito taking an additional blood meal on the speed of parasite development. In natural populations, Anopheles gambiae females bite humans to acquire nutrients for each round of egg development, of which there can be several. This multiple feeding behavior is challenging to reproduce in laboratory experiments with Plasmodium-infected mosquitoes, limiting our understanding of its potential effects on malaria transmission, and yet this much more closely resembles the situation occurring in field populations.
An additional blood feed accelerates parasite development and increases malaria transmission potential
In our study, providing a second blood meal to female mosquitoes 3 days after they became infected with the malaria parasite Plasmodium falciparum dramatically increased the size of developing parasites and accelerated the appearance of transmissible forms in the salivary glands, shortening the EIP by 2.3 days (21%) and making these mosquitoes infectious sooner. The increased available resources, particularly amino acids and lipids, provided by an additional blood meal are apparently accessible to and potentially taken up by the parasite. Plasmodium therefore has a remarkable plasticity to use available mosquito nutrients to accelerate its development. Indeed, similar effects of double blood feeding are reported in several mosquito-pathogen interactions so this may be a more generalized mechanism for parasites to increase their chances of transmission to the next host.
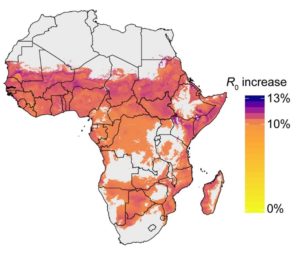
Incorporating our findings into a mathematical model across sub-Saharan Africa reveals that the malaria transmission potential, a parameter known as R0, incorporating multiple blood feeding is likely to be higher than previously thought . Since natural mosquito populations that are already feeding multiply, this suggests the contribution of multiple blood feeding as a driver of transmission is currently underestimated and elimination in these endemic areas may be more challenging than anticipated.
What are the implications for controlling malaria?
Our data suggest that, firstly, parasites can be transmitted by younger mosquitoes, which are known to be less susceptible to insecticide killing, with possible negative effects on the efficacy of currently available insecticide-based strategies (bed nets and indoor residual sprays).
Secondly, strategies encouraging mosquitoes to feed on nearby animals rather than people (zooprophylaxis) may not be as effective as expected, because animal blood, while nutritionally different to human blood, may still permit accelerated development within mosquitoes, increasing the likelihood of females transmitting parasites when they do feed on people.
Finally, control strategies that interfere with egg production to reduce Anopheles populations, such as genetically engineered population suppression gene drives, may increase the resources within the mosquito available to parasites, accelerating their development and inadvertently favoring malaria transmission.

Comments