In an article published in Breast Cancer Research, Charles Dumontet’s team from the Cancer Research Center in Lyon show that the adipose tissue surrounding breast cancer increases resistance to trastuzumab (or Herceptin), a therapeutic monoclonal antibody targeting the oncogene HER-2. Fatty tissue’s crimes on cancer continue to mount up, yet, it seemed so innocent to begin with!
Adipose tissue: Under a seemingly docile exterior lies a highly active tissue
At first glance, adipose tissue seems to be a rather monotonous tissue, comprised mostly of adipocytes, fat-storing cells. These globular cells contain a single large lipid vacuole (made up of triglycerides).
Their main function is to maintain an energy balance by storing excess fat and releasing it when needed in the form of fatty acids. However, an adipocyte is not only a passive “fat reservoir”. We now know it has other very active functions: it produces and secretes many biomolecules such as hormones, growth factors and proinflammatory cytokines, a group of molecules named adipokines.
Adipose tissue also contains other cells that are less abundant than adipocytes and barely visible on tissue sections. Among these other cells there are fibroblasts, endothelial cells and macrophages as well as adipocyte progenitors (adipose-derived stem cells, preadipocytes) that support the renewal of adipose tissue.
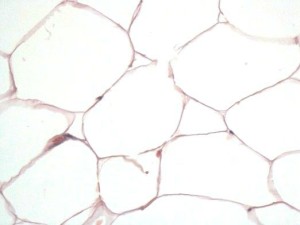
Histological examination of normal mammary adipose tissue after Hematoxylin-Eosin (HE) staining (original magnification X 400). Note that mature white adipocytes have a single lipid droplet (in white), which essentially occupies the fat cell volume surrounded by a reduced rim of cytoplasm. © Ghislaine Escourrou
Adipose tissue and breast cancer: A dangerous dynamic duo
In the breast, adipose tissue is particularly abundant and surrounds the mammary glands. Thus, when breast cancer becomes invasive, it comes into direct contact with fatty tissue. Recently, numerous studies have shown that adipocytes and their progenitors promote breast cancer aggressiveness by stimulating proliferation and, especially, invasion by secreting proteases, pro-inflammatory cytokines and by modulating cancer cell metabolism.
In obesity, not only the size and number, but also the secretions of adipocytes are profoundly impacted. Obese breast cancer patients exhibit at diagnosis more aggressive tumors and disease progression is marked by a much higher rate of mortality. Hence, studying the effect of fat cells on disease progression is of a major interest in medicine, especially for the treatment of obese patients.
The current paper presents a compelling hypothesis on the role adipose tissue may play in response to anti-cancer treatments, protecting tumor cells from their toxic effects. This may explain the decreased survival rates observed in obese breast cancer patients despite supposedly adequate treatment plans.
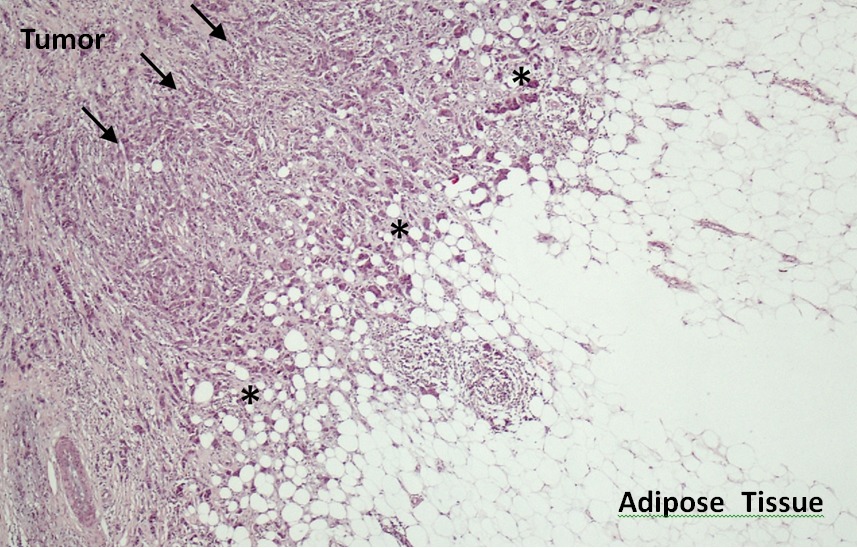
In breast cancer, early local tumor invasion results in immediate proximity of cancer cells to adipose tissue. Histological examination of an invasive breast tumor after H&E staining (original magnification X 200). Arrows indicate the tumor. Note that at the invasive front, tumor and adipose tissue are in contact (indicated by stars). © Ghislaine Escourrou
Trastuzumab, a targeted weapon thwarted by fat cells
In recent years, efforts have been made to develop targeted therapeutics for cancer treatment. This type of therapy specifically targets abnormalities contributing to the growth and spread of cancer by specifically eliminating or blocking the growth of tumor cells, as opposed to traditional therapies that damage both normal and cancerous cells.
Trastuzumab (Herceptin ™) is part of this family and has shown extraordinary results in breast cancer treatment, when administered alone or in combination with chemotherapy. It is an antibody directed against HER2 receptor, which is over-expressed by breast cancer cells in approximately 20% of patients.
Despite the remarkable efficacy of this drug, some patients are resistant to this treatment either directly from the first administration, or after some time of exposure to the drug. Now, understanding the underlying mechanisms inducing this resistance is essential to further improve the efficacy of this treatment.
In this work published in Breast Cancer Research, Charles Dumontet’s team shows that fat cells decrease trastuzumab effectiveness using a variety of experimental approaches including mice models. Through secretion of soluble factors (whose precise nature remains to be identified), fat cells activate survival mechanisms in recipient tumor cells diminishing the effect of the drug.
This study is very original in many respects. On the one hand, it shows, for the first time, that fat cells can have a negative effect on the efficacy of targeted therapies. The link between obesity and resistance to trastuzumab remains poorly explored, but this paper highlights the need for clinical studies to clarify this.
On the other hand, this work strongly suggests that the survival mechanisms activated in tumor cells by surrounding adipose tissue could also counteract the effects of other types of anti-cancer therapies, such as chemotherapy. This sheds new light on the role of fat cells in breast cancer progression and could contribute to the development of new more effective therapies, especially for the treatment of obese cancer patients.
Comments