
Human giardiasis is a globally important intestinal parasitic disease caused by the eukaryotic protozoan Giardia duodenalis (syn. Giardia intestinalis, Giardia lamblia). This parasite is primarily considered a waterborne pathogen that has a direct faecal-oral life cycle. As such, only a definitive host is required for effective transmission.
Whilst cosmopolitan in distribution, prevalence of human giardiasis can be particularly high in rural areas of low- and middle-income countries (LMICs) where adequate water, sanitation, and hygiene (WASH) infrastructure is lacking, including those in sub-Saharan Africa. In these areas, human giardiasis predominantly afflicts children < 15 years of age, which can severely impact childhood development. Of the eight known morphologically identical but genetically distinct assemblages of G. duodenalis, almost all human infections are caused by assemblages A and B. Interestingly, however, recent evidence suggests there may be marked differences in the types and degree of pathology experienced by those infected with either G. duodenalis assemblages A and B, as well as between those burdened with single-assemblage (A or B) or mixed-assemblage (A and B) infections.
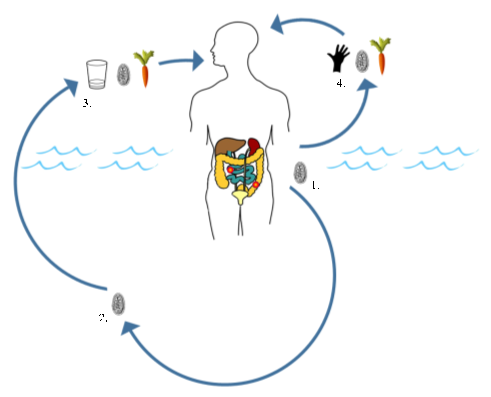
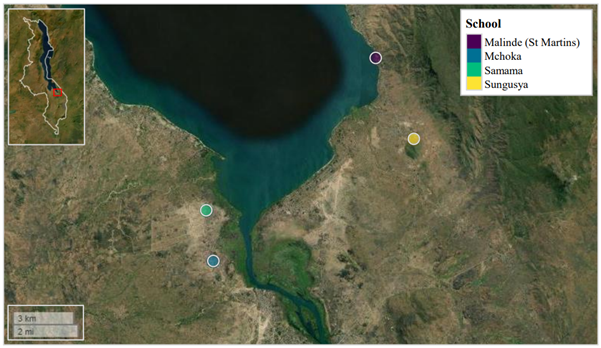
We investigated this in a recent publication, where primary school children attending four schools in close proximity to the southern shoreline of Lake Malawi (Mangochi District, Malawi) were surveyed.
Lake Malawi is one of seven African Great lakes. With a water surface area of ~29,600 Km2, the lake occupies approximately one-fifth of Malawi, whilst also bordering Tanzania and Mozambique along its eastern shoreline. The lake’s most southern shoreline borders Mangochi District. Owing to insufficient WASH infrastructure, many members of Mangochi’s multiple communities rely on the lake as a source of drinking water, food (via fishing), a place to bathe and tend livestock, and for recreation. As such, human water-contact with the lake’s shoreline is commonplace, resulting in a high prevalence of waterborne diseases in this area, such as urogenital and intestinal schistosomiasis.
Methodology
Stool samples were provided by 305 school-aged children. A proportion of each stool was used to carry various point-of-care diagnostic analyses including Kato-Katz faecal microscopy and QUIK CHEK rapid diagnostic tests (RDTs) to diagnose infection with giardiasis, and faecal occult blood/faecal calprotectin RDTs to assess the presence of overt blood and calprotectin within the stool (a measure of intestinal pathology). A proportion of each stool was also stored in ethanol for transportation to the Liverpool School of Tropical Medicine (LSTM) for future DNA extraction and molecular assessment. Intestinal pathology data were also collected via questionnaire; each study participant that provided stool also responded to the questions ‘do you currently have abdominal (stomach) pain?’ and ‘do you currently have loose stool (diarrhoea)?’.
Back at the LSTM, DNA was extracted from all faecal samples using a bead-beating step to liberate DNA from any potential helminth (worm) eggs and protozoan cysts. A diagnostic 18S real-time PCR was then carried out to measure prevalence.
Results and perspective
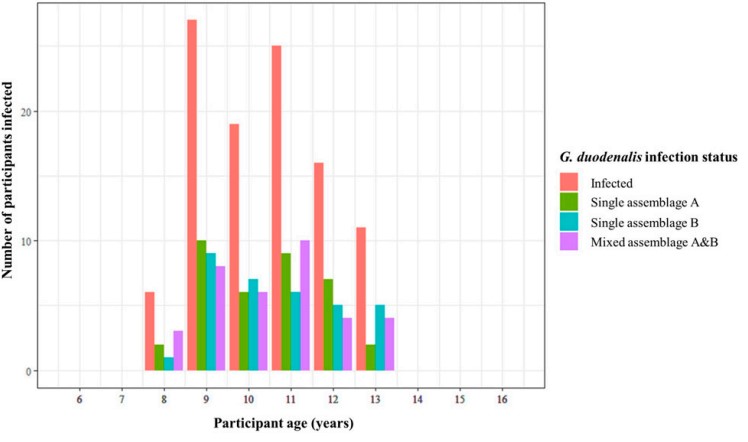
The diagnostic real-time PCR showed the prevalence of G. duodenalis infection to be 39.3% within this study cohort, a fairly typical prevalence of giardiasis infection in settings such as these. Using all samples positive for G. duodenalis infection (120), an endpoint nested PCR targeting the G. duodenalis beta-giardin (bg) gene was then performed (successful in 108 of these 120 samples). The amplified bg fragment was sequenced from all 108 samples, meaning the infecting G. duodenalis assemblage(s), (A, or B, or both!), could be determined.
We found a fairly equal prevalence of infection types across all G. duodenalis infections (G. duodenalis assemblage A only: 35%, assemblage B only: 32%, and infection with both assemblages A and B: 33%). This came as a bit of a surprise, as in previous studies of this kind (of which there are few), a much higher prevalence of one assemblage (A or B) over the other is typically found. This led theorisation that any discordance in either assemblage being more (or less) associated with intestinal pathology is not a result of one or the other assemblage type being more pathogenic organisms, but rather that it is perhaps the least prevalent assemblage within a given locality that is responsible for causing intestinal pathology.
Interestingly, despite a fairly equal prevalence of infection types, we found a statistically significant positive association between single infection with G. duodenalis assemblage B and both self-reporting of stomach pain and self-reporting of diarrhoea, but no association between single infection with G. duodenalis assemblage A and any form of intestinal pathology.
With regard to self-reported diarrhoea specifically, these data are in agreement with some previous studies that also found an association between single infection with G. duodenalis assemblage B and symptomatic diarrhoea (but no such association between single infection with G. duodenalis assemblage A and diarrhoea) yet are also in disagreement with other studies that reached the opposite conclusion.
This is also true for self-reported stomach pain; these data are in agreement with some previous studies that have also found an association between single infection with G. duodenalis assemblage B and stomach or abdominal pain (but no association between single infection with G. duodenalis assemblage A and stomach/abdominal pain), but disagreement with other studies that have again reached the opposite conclusion.
No association was found between any G. duodenalis infection type and faecal-occult blood or faecal calprotectin. These assays, however, were deemed poor diagnostic markers of infection with G. duodenalis when compared to the real-time PCR and QUIK CHEK RDTs.
We also found a positive association between mixed infection with both G. duodenalis assemblages A and B and self-reporting of diarrhoea, as well as a statistically significant association between mixed infection with both assemblages and self-reporting of stomach pain. Although interesting observations, conclusions as to whether these associations are a result of individuals being infected with G. duodenalis assemblage B (potentially more pathogenic within this study group), or whether there is interplay between mixed infection with both assemblages and intestinal pathology, cannot be drawn using these data. As such, additional work is needed to scrutinise further whether infection with both human-infecting G. duodenalis assemblages (A and B) has a more detrimental impact on intestinal health than infection with just one assemblage.
We concluded that molecular surveillance methods such as these should continue to be used, particularly in areas lacking adequate access to WASH infrastructure, to better understand the detrimental health impacts, not only of infection with G. duodenalis but also of poor access to clean and safe drinking water and sanitation facilities. In doing so, not only can the pathological impact of human giardiasis be better understood, but, through improved disease surveillance, control, and access to WASH infrastructure, a reduction in disease-associated pathologies experienced by the world’s most disadvantaged communities can also be achieved.
We would like to thank all study participants attending Samama, Mchoka, Sungusya, and Malinde (St. Martin’s) schools for their generosity during sample collections, as well as all teachers and health workers in Mangochi District for their assistance during sample collections and study planning.

Comments