
 As we come to the end of April, and World Malaria Day on 25th April, many of us are reflecting on what progress has been made in the last 12 months (and historically) towards eradicating the disease. The theme for this year’s World Malaria Day is ‘Time to deliver zero malaria: invest, innovate, implement’.
As we come to the end of April, and World Malaria Day on 25th April, many of us are reflecting on what progress has been made in the last 12 months (and historically) towards eradicating the disease. The theme for this year’s World Malaria Day is ‘Time to deliver zero malaria: invest, innovate, implement’.
We have of course employed many different strategies to control and eliminate the disease over the years. However, the parasite and its mosquito vectors are developing resistance or changing to evade control measures. We therefore need to continuously look for new tools and control measures if we are to achieve zero malaria.

For the last few decades, pathogenic fungi have been used as biopesticides to control insect pests, and specifically Beauveria spp. and Metarhizium spp. have been used to control mosquitoes. But, resistance against these fungi is emerging already, so there is a need to find new entomopathogenic microorganisms. And it seems as though many of the likely candidates could be underneath our feet in the soil.
In their paper, Edounou Jacques Gnambani and colleagues describe the entomopathogenic properties of a soil bacterial isolate from the Chromobacterium genus (preliminarily named Chromobacterium anophelis sp. nov. IRSSSOUMB001) that is native to Burkino Faso. As the authors’ previous study had already shown its virulence properties against adult Anopheles coluzzii mosquitoes, this study specifically tested the bacterial isolate’s virulence against larvae, its impact on the reproductive fitness of infected mosquitoes, and mosquito body size of progeny.
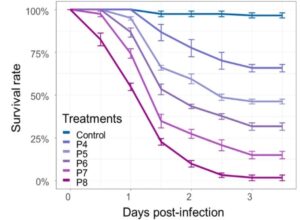
Mosquito larvae from a population that were known to be highly resistant to insecticides (96.9% of the mosquitoes used in the study carried the kdr resistance gene) were mixed in solutions containing different concentrations of Chromobacterium anophelis sp. nov. IRSSSOUMB001. 80% of larvae put into the solutions containing the higher bacterial concentrations were killed in 3 days, but lower concentrations of the bacterial solution were not so effective and killed only 25-50% of the larvae by the end of the 3 days.
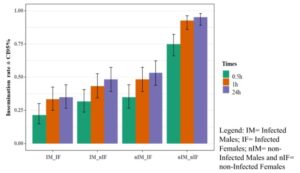
To measure reproductive fitness, male and female mosquitoes were fed glucose solutions containing Chromobacterium anophelis sp. nov. IRSSSOUMB001. Insemination rates were reduced to 35% when males and females were exposed to the bacteria compared to non-infected mosquitoes (95% insemination rate). Infecting only the male or female mosquito reduced insemination rates to about 50%.
Progeny wing size – the proxy for body size – was measured from mosquitoes reared from infected and uninfected female mosquitoes. Progeny wing size was significantly reduced when a mosquito was infected with the bacteria. Female wing size dropped from an average of 2.55mm to 2.1 mm in mosquitoes born from infected mosquitoes. For males, the average wing size dropped from 2.44 mm to 1.99 mm.
Chromobacterium anophelis sp. nov. IRSSSOUMB001 clearly displays strong virulence against larvae, impacts the reproductive fitness of the adults and reduces the body size of the progeny. The mechanisms behind the pathogenicity are not known yet, so this has to be investigated further. Also, high concentrations of the bacteria are needed to effectively kill the larvae, which might impact the microbial ecosystem of the soil or environment where bacterial solutions are used. Resistance against this bacteria also has to be considered and whether this isolate could be used in conjunction with other entomopathogenic microorganisms or indeed other control measures to reduce the possibility of resistance emerging.
It is however encouraging to know new control measures are being investigated, and that the soil could hold potentially hundreds of new and useful entomopathogenic microorganisms.

Comments