
Schistosomiasis
Schistosomiasis is a neglected tropical disease (NTD) caused by parasitic flatworms. The disease affects over 200 million people and causes thousands of deaths annually. Circa 95% of human infections occur in sub-Saharan Africa where Schistosoma mansoni and S. haematobium are responsible for intestinal and urogenital schistosomiasis, respectively.
The control of schistosomiasis requires the interruption of a complex lifecycle. There are several points of the lifecycle that can be targeted to control the disease which include snail control, chemotherapy, health education, improved access to clean water, sanitation and hygiene.
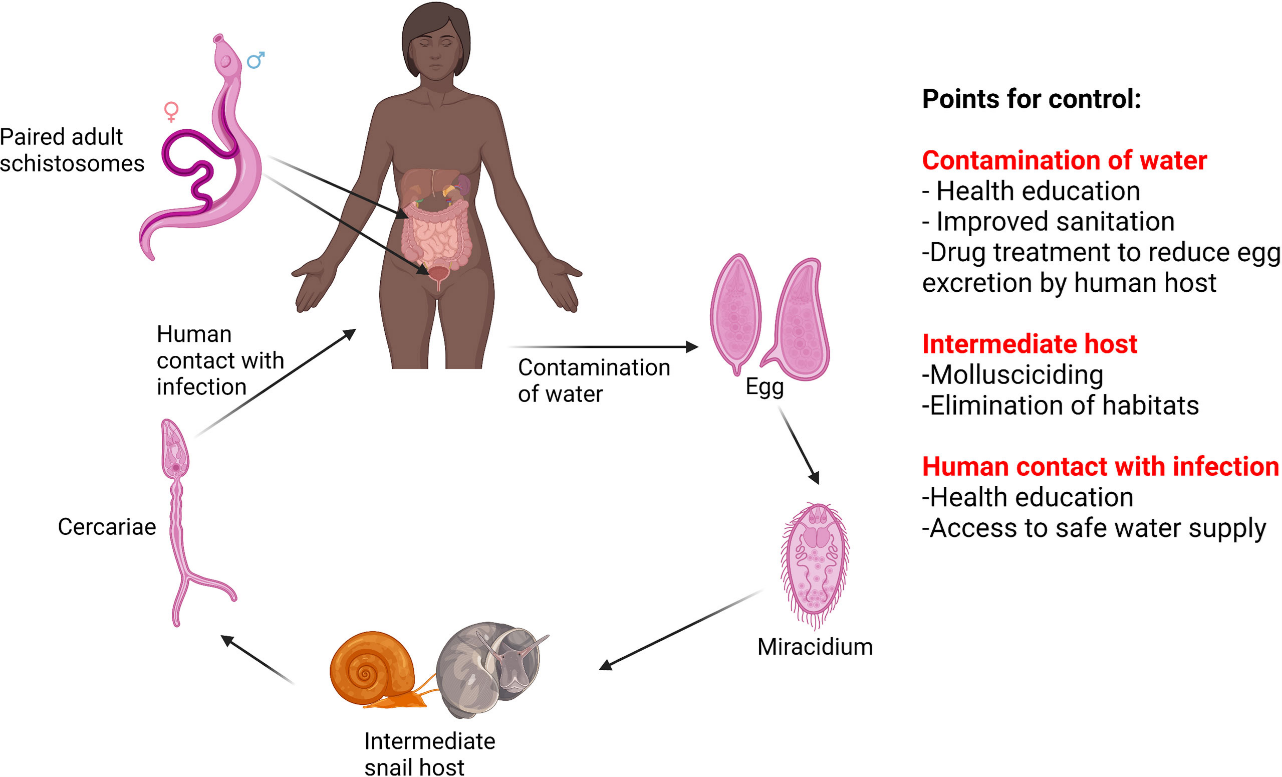
Schistosomiasis treatment
Schistosomiasis can be treated with praziquantel, a broad spectrum antihelmintic developed in the 1970s and is the only drug available for treatment. Praziquantel is effective against all Schistosoma species infecting humans and is delivered through large scale mass drug administration (MDA) programs in sub-Saharan Africa. In recent years the access to praziquantel has improved primarily through the pledge from Merck KgaA to donate 250 million praziquantel tablets to endemic countries annually. This has significantly aided in reducing morbidity and infection intensity.
Despite annual MDA of praziquantel for two decades, hotspots of unabated transmission and associated morbidity persist, and some areas have experienced re-emergence of the disease where it was previously controlled. It is not clear why this could be but a range of social, environmental, parasitological and drug factors may be at play. For example, praziquantel is not a perfect drug – it does not prevent re-infection, and its efficacy is stage and sex dependent (schistosomes have male and female forms). Annual praziquantel treatment is effective in reducing morbidity, but has been shown to be insufficient in curing all treated individuals in a single dose.
Genomic surveillance of Schistosoma populations is required alongside control efforts to determine how parasite populations are evolving in response to control measures and why hotspots persist.
The new WHO guidelines on control and elimination of human schistosomiasis
The new NTD roadmap and guidelines on the control and elimination of schistosomiasis by the W.H.O sets out an ambitious control strategy to eliminate schistosomiasis as a public health problem by 2030 and to achieve interruption of transmission in selected countries. Large scale MDA programs remain at the forefront of the control strategy.
Historically school aged children have been the focus of MDA programs, but the new treatment guidelines aim to extend the eligibility for praziquantel treatment to all age groups above the age of two. The expanded access to praziquantel treatment aims to accelerate the path to schistosomiasis elimination, however; they also pose an increased risk of the development of praziquantel resistance.
Drug resistance in schistosomiasis
The antischistosomal properties of praziquantel were discovered in the 1970s, but its mode of action and mechanism of resistance in S. mansoni has remained a significant enigma in schistosomiasis research despite research efforts. The reliance on a single drug, praziquantel, to treat a disease of this magnitude is worrying, should resistance develop.
Praziquantel resistance can be readily selected for in laboratory strains of S. mansoni and several field reports indicate reduced efficacy of praziquantel due to low cure rates. Suggestions of praziquantel resistance in the field remain controversial, as these reports are likely explained by factors other than drug resistance; for example, rapid re-infection and inadequate dosing.
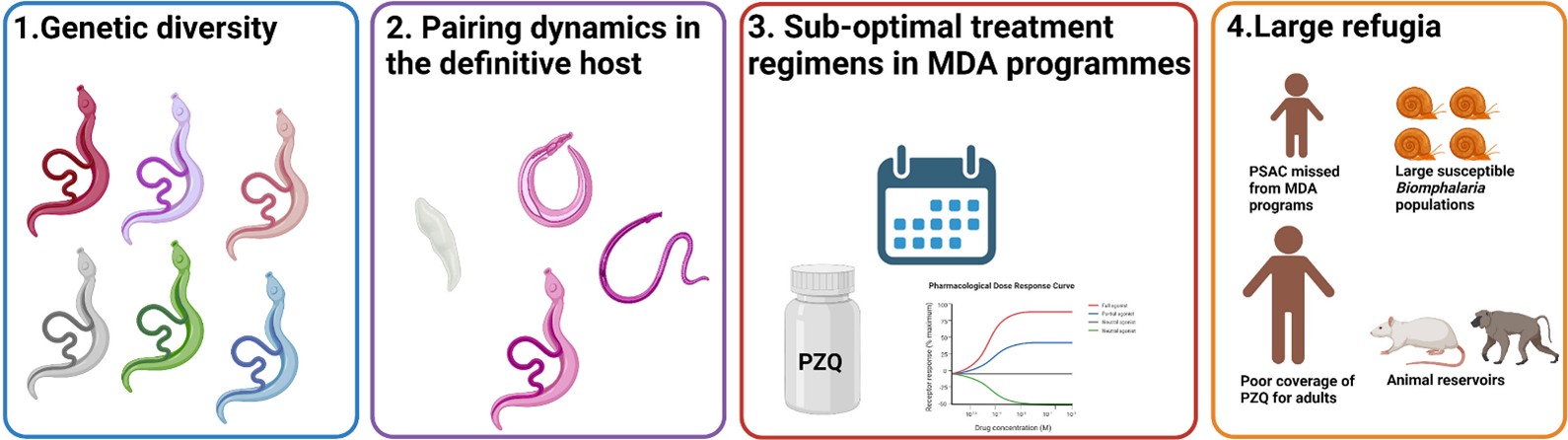
A recent review discusses praziquantel resistance within the context of confounding factors in endemic field settings. The review concludes there is insufficient evidence for the emergence of drug resistant S. mansoni populations within field settings. Four key factors are proposed, which may explain the lack of praziquantel resistance observed in field S. mansoni populations; the extensive genetic diversity, complicated pairing dynamics of schistosomes, sub-optimal treatment regimens and large refugia.
SmTRPM-PZQ a key determinant of praziquantel sensitivity
Recent advances have revealed for the first time a molecular target of praziquantel in Schistosoma mansoni, called the transient receptor potential melastatin praziquantel channel (SmTRPMPZQ). SmTRPMPZQ has been implicated in the mode of action and in praziquantel drug resistance in S. mansoni by two independent research groups using different approaches. When S. mansoni is exposed to praziquantel, the ion channel opens, causing a large influx of calcium ions into the schistosome, resulting in paralysis and worm death.
Park et al. described how praziquantel binds to SmTRPMPZQ and mapped the drug binding site in the channel. Using targeted mutagenesis, the authors identified mutations in SmTRPMPZQ which can result in decreased or a complete loss of praziquantel sensitivity.
Le Clec’h et al. ran a genome-wide association study (GWAS) to determine if there were specific genetic regions in S. mansoni which differed significantly between pools of praziquantel resistant and praziquantel sensitive worms. SmTRPMPZQ was found to be significantly associated with the extreme praziquantel resistant phenotype in S. mansoni (377 times more resistant than the sensitive phenotype). The praziquantel resistant S. mansoni strain demonstrated recessive inheritance, meaning only homozygotes carrying two copies of the SmTRPMPZQ 741987C allele survived praziquantel treatment.
Together these studies provide insights into how praziquantel works, and provide a framework for large scale drug resistance monitoring in field Schistosoma populations. Genotyping of SmTRPMPZQ from natural S. mansoni populations could provide valuable insights into the genetic variation within this gene to see if praziquantel resistance is a problem, and should be a primary focus moving forward.

Comments