
During the first few days of the ongoing COVID-19 pandemic in mid-to-late March of 2020, a curious thing happened. Public places that are usually quiet were suddenly flooded by people aiming to spend their unexpected free time enjoying the good weather outdoors. This timing coincides with the start of the spring season when ticks, such as the Rocky Mountain wood tick (Dermacentor andersoni), also come out of their diapause and start questing.
Ticks, being efficient ectoparasites, don’t care about the pandemic ravaging our population and economy. They continue to go about their business, questing for hosts, taking blood meals, molting from larvae to nymph to adults, mating, laying their eggs and dying. Although ticks may actually prefer a non-human mammal host, ticks can transmit a variety of pathogens to humans that can cause debilitating diseases. Therefore, it is important to predict and control ticks and the diseases they transmit.
One of the most important non-human hosts for ticks are rodents. Rodents provide blood meals for almost all larval and some nymphal development of various species. Rodents are also important in the ecology of many tick-borne pathogens, serving as the reservoir or amplification host for them. Given their importance, rodent density has been proposed to be an important driver of tick density and tick-borne disease risk.
For example, in North America rodents (and their food source, acorns) are a good predictor of Lyme disease risk. However, experimental studies to investigate the causal relationship between rodent density, tick density and tick-borne disease risk have been lacking, allowing only a correlative analysis.
This knowledge gap was recently addressed by Aleksandra Krawczyk and her colleagues in the group of Willem Takken at Wageningen University. The researchers manipulated rodent density in six 50 by 50 m plots each in two forest reserves in the Netherlands. In four plots acorns were added to feeding stations in November and January of 2012 and 2013. In four other plots rodents were removed once a month by trapping and euthanizing them between September 2012 and December 2014. In the last four plots there was no acorn addition or rodent removal, but all other elements, such as empty feeding stations and a plastic screen barrier, were installed around the plots. The researchers trapped small mammals every 3 months from September 2012 to December 2014 and estimated rodent density. Ticks were collected by dragging between January 2013 and January 2016. Ticks and a small ear biopsy were also collected from the captured rodents. DNA was extracted from both ear biopsies and ticks and tested for a range of tick-borne pathogens using multiplex RT-PCR. Finally, a range of statistical models was used to evaluate whether or not the experimental treatments had an effect on rodent density, and whether or not changes in rodent density impacted tick density or tick-borne pathogen prevalence in current or subsequent years.
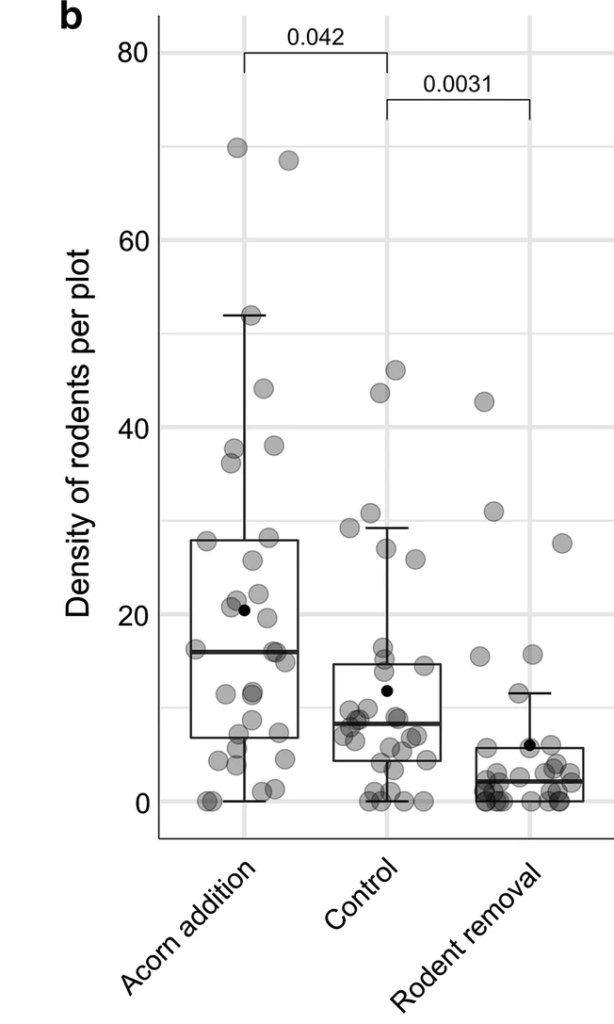
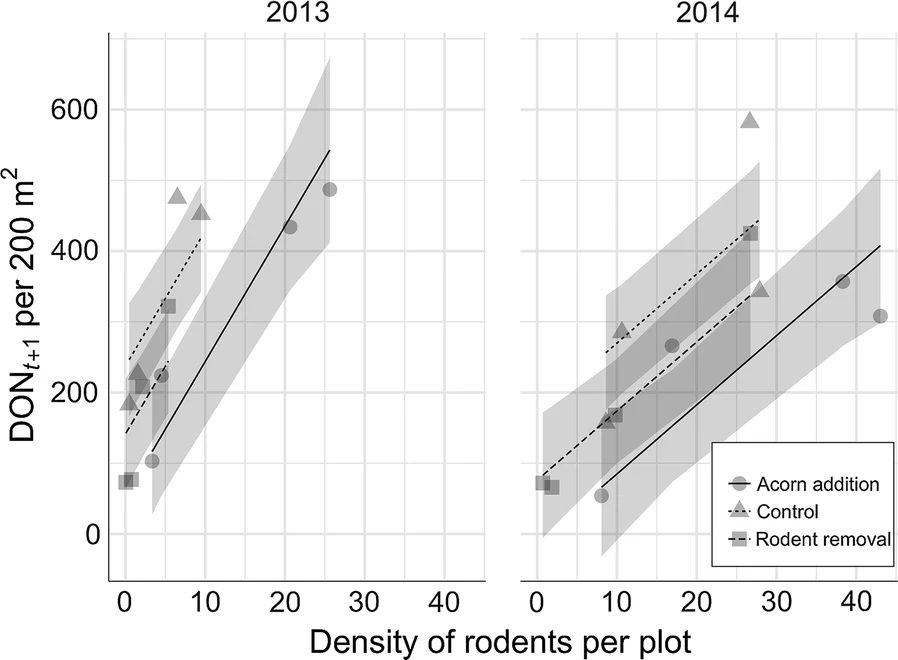
The authors found that wood mice and bank voles were the main rodents inhabiting the plots and were parasitized by Ixodes ricinus ticks. Rodent removal significantly decreased bank vole density, while acorn addition significantly increased it relative to the control plots in 2013 and 2014, with acorn addition leading to a higher density of wood mice in the second year of the study. Acorn addition did not affect the density of nymphs (DON) but rodent removal significantly reduced it in the same or following year. There were many more larvae and nymphs found on wood mice relative to bank voles. Rodent density was significantly positively associated with the density of nymphs in the following year, explaining 61% of the variation in tick density.
A smorgasbord of tick-borne pathogens was detected in rodent ear biopsies and questing nymphs, including some horizontally transmitted with rodent reservoirs (e.g. Babesia microti, Borrelia afzelii, Neoehrlichia mikurensis); some vertically transmitted with rodent reservoirs (Borrelia miyamotoi, Spiroplasma ixodetis); and some horizontally or vertically transmitted pathogens with bird reservoirs (Borrelia garinii and Rickettsia helvetica).
For B. afzelii and N. mikurensis, rodent density was positively associated with nymphal infection prevalence (NIP) and the density of infected nymphs (DIN) in the following year. Nymphal infection prevalence of B. microti was negatively associated with rodent density, while the density of infected nymphs had a non-linear association with rodent density. For the bird-associated pathogen B. garinii, the authors found a negative association between NIP and rodent density, and no association between DIN and rodent density. For the vertically transmitted pathogens, the associations with rodent density was equivocal, with no effect between rodent density and NIP for R. helvetica, and a positive effect between DIN of the same pathogen. For B. miyamotoi, the association between rodent density and NIP was negative in 2013 and positive in 2014, while for DIN it was significantly positive for both years. Finally, there was a significant negative association between rodent density and the NIP for S. ixodetis, while a non-linear association was detected for the DIN of the same pathogen.
The authors of this study were therefore able to experimentally manipulate rodent density in plots. They also showed that increasing rodent density leads to increased tick density in subsequent years. However, considerable variation in tick density was found between the plots that received the same experimental treatments, such that plots with different treatments did not have significantly different tick density. This suggests that simply manipulating rodent density (e.g. by large scale rodent control) would not necessarily reduce tick density in a consistent manner. This may be due to the importance of other hosts as well as abiotic factors, such as temperature.
In terms of tick-borne disease risk, the authors found an overall positive association between rodent density and the NIP and DIN for horizontally transmitted pathogens utilizing rodents as reservoir or amplifying hosts, an overall negative association for the NIP and DIN of horizontally transmitted pathogens utilizing birds as reservoir hosts, and inconclusive results for vertically transmitted pathogens. These results suggest that reducing rodent density, partly by reducing tick density, would likely also reduce the transmission risk of horizontally transmitted rodent-borne pathogens, but might increase the transmission risk for horizontally transmitted bird-associated pathogens. However, the models only explained a modest amount of variation in NIP and DIN for many of these pathogens, so one has to be careful not to over-interpret the results. There are likely many other factors impacting the transmission risk of these tick-borne pathogens.
Overall, the authors succeeded in providing experimental evidence for the importance of rodent density for tick density and partially to tick-borne disease risk, in support of earlier correlative studies. However, it would be premature to use the results of this study to design large-scale rodent control interventions to reduce tick density and tick-borne disease risk. In fact, the research shows that any such interventions would have to contend with large uncertainty in the expected results, in addition to differential impacts on various tick-borne pathogens, partly depending on their transmission modes. While rodent density is clearly important, it is just one of the many factors determining the complex ecology of these ticks and the diseases they transmit. Therefore, we should be careful when venturing outdoors, as protective clothing and checking for ticks remains best practice to protect ourselves from these parasites and their pathogens.

Comments