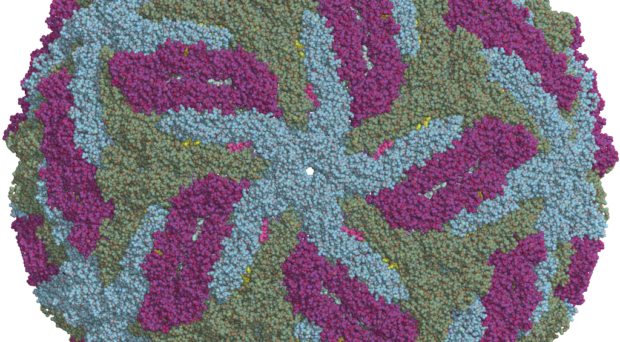
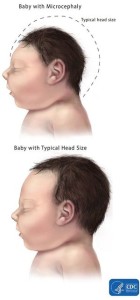
Until Zika swept through the Americas in 2015, the virus had gone relatively unnoticed by the world. Previous outbreaks in Micronesia and Polynesia in 2013-2014 did not report such serious symptoms- specifically microcephaly- and so it was suggested that perhaps a more virulent strain of Zika was circulating in the Americas. It was also possible that herd immunity in these populations could have played a role in reducing the virulence, since the Asian-lineage strain of Zika- from which the South American virus originates- is thought to have been circulating around Asia for around 10 years. Re-analysis of the Asian outbreaks in fact revealed a 1% rate of microcephaly, still- much lower than the rates of congenital malformations in South America. For more informed policies around the prevention and treatment of Zika, the virulence factors of this virus need to be better understood.
Yuan et al (2017) have published a study into the effects of sequence mutations in genes encoding Zika proteins. Using reverse genetics and mouse neurovirulence models to validate their findings experimentally, they have decoded an important mutation in the Zika M-protein that is significantly associated with microcephaly. In-vivo comparisons of circulating contemporary Zika strains and their Asian ancestral strain found them to be much more virulent than their predecessor.
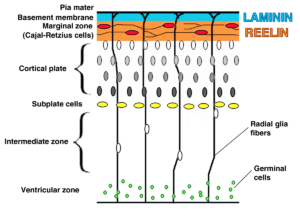
Mortality amongst neonatal mice injected (intra-cerebrally) with the contemporary strains was 100% with several neurological manifestations, compared to just 16% with the ancestral strain. Through sequencing these strains, numerous substitutions were found. Selecting seven of these mutations, they constructed new viral particles containing these to determine their impact on neurovirulence. The results showed striking effects from a Serine to Asparagine mutation at residue 139 (S139N). This mutation enhanced replication of the virus in human and mouse neural progenitor cells (NPC)and caused more extensive cell death. In an embryonic microcephaly model, the mutant virus caused cell death in the cortical plate and thinning of the cortex, as well as enhanced infection of and replication in the NPCs resulting in severe microcephaly. Most remarkably, these neurovirulent effects could be ameliorated in the original parent virus (where the substitution was first uncovered) with the reversal of mutant to a N139S genotype.
Estimating, viral load of a natural infection is always difficult. Here the authors use 10 plaque forming units (PFU) of the various viruses to inoculate mice intra-cerebrally. One study reports that regardless of administration route- lethal doses of Japanese Encephalitis Virus (JEV) were the same in mice. Recently Dowell et al, developed an A129 (immunodeficient) mouse model to simulate a more natural infection (by subcutaneous inoculation) with Zika. They do use a much higher dose of Zika (106 PFUs/inoculum) and so it would be interesting to look at dosage effects in this model too. Similarly, for modelling purposes it would be useful to know how these contemporary strains and different viral loads affect vectoral capacity/ fitness in Ae. aegypti. In the closely related Dengue Virus, residue 139 on the M-protein is essential for viral maturation, egress and secretion. Yang et al suggest that the S139N might affect maturation of Zika virus, which could have knock-on effects in terms of viral fitness.
In terms of epidemiology, modelling of gene variants estimates the S139N mutant to have arisen between November 2012 and October 2013. Its occurrence before the 2013 outbreak in French Polynesia correlates with the emergence of microcephaly reports, as well as other abnormalities such as Guillain-Barre syndrome. These findings help to explain Zika’s transformation from an overlooked arbovirus to a pathogen of global significance. More and more we are gaining a better understanding of the virulence, immunology, transmission routes and even vectoral competence of Zika virus. Furthermore, these data could also feed into modelling outbreaks of other closely related arboviruses.

Comments