
Schistosomiasis is a major public health problem in 52 endemic countries. With hundreds of thousands of people at risk of infection with urogenital and intestinal species of schistosomes, it not only causes death but also high levels of morbidity, stunting of growth and can impair cognitive development, leading to loss of school/work days; and therefore not only has severe health implications but additionally economic ones.
The lifecycle of schistosomiasis is complex involving two free-living, non-feeding larval stages (miracidia and cercariae), a specific intermediate freshwater snail host and a definitive host (generally mammalian).
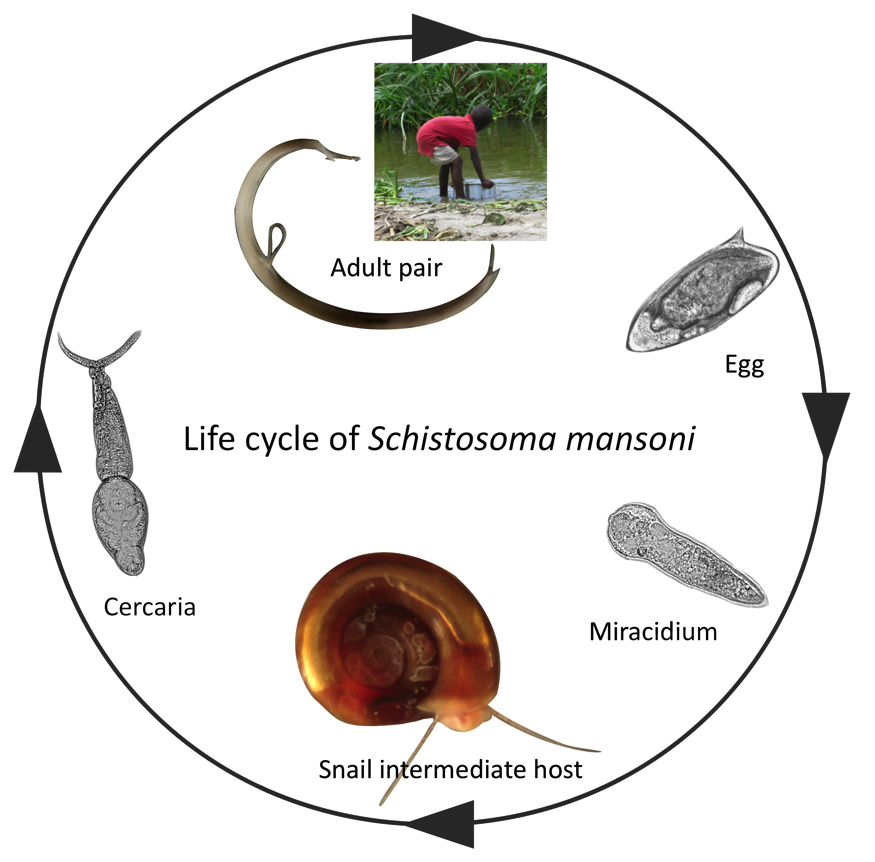
Because of this complexity the study of the snail hosts, as well as the human host, are essential for the push towards control and elimination of this neglected tropical disease. A key area of research is the mechanism of infection, and subsequent host-parasite interactions that can lead to parasite reproduction in the snail host. The physiological and behavioural changes occurring in the snail host after infection can determine the success or failure of the parasite’s infection attempt yet the molecular components and mechanisms underpinning these changes remain largely unknown.
In a recent study published in Parasites and Vectors journal by Wang et al, researchers looked at the snail Biomphalaria glabrata ganglia nervous system before and after infection with Schistosoma mansoni miracidia to determine which physiological changes occur in the snail after infections with schistosomes.
The central nervous system (CNS) of snails is made up of several ganglia that control metabolic activities in all of the snail’s tissues and organs, for example in the reproductive organs and the head/foot (or exposed part) of the snail. Neuropeptides, small protein-like molecules that act as chemical transmitters, are used to direct growth, reproduction and immunity in the host snail and are predominantly found in the CNS.
Wang et al used mass spectrometry and nano-HPLC (high performance liquid chromatography) methods of fractionation, to determine which CNS neuropeptides within B. glabrata were modified after infection with S. mansoni miracidia and after sporocyst (the developing parasite within the snail) formation.
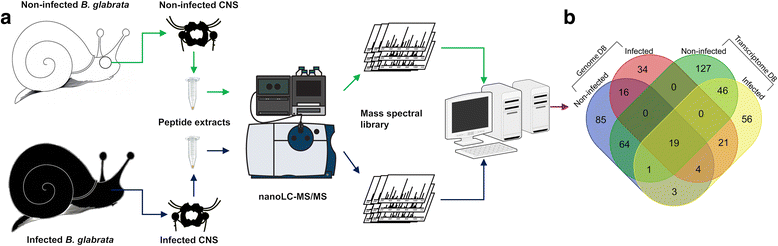
They started by identifying which neuropeptides where present in non-infected and 12-day infected (prepatent) snails, and then created a protein-by-protein interaction network to determine which proteins from the snail and the parasite where present and interacting in infections. Finally they compared neuropeptide abundance, particularly those involved in snail reproduction, in non-infected and infected snails.
The development of the sporocyst within the snail has a huge impact on the immunity and metabolism of the snail intermediate host, potentially leading to energy and space resources being diverted to the parasite rather than the snail. In this host-parasite interaction system successful parasite sporocyst development leads to the castration of the snail by suppressing the maturation of gonads, meaning that infected snails support the production of cercariae rather than using resources to produce their own offspring. This is why the authors of the study particularly focused on neuropeptides involved in snail reproduction, in order to determine the components that drive this post-infection physiological and behavioural change in the snail host.
The authors found that the neuropeptides and precursor proteins, particularly those involved in snail reproduction were heavily down regulated and less abundant in prepatent snails compared to non-infected snails, suggesting that this could be the mechanisms for physiological castration of Biomphalaria by schistosome infections. Other neuropeptides that appeared down regulated in prepatent snails were linked to snail feeding and growth. Conversely a group of proteins associated with higher metabolic rates in molluscs were more abundant in prepatent snails.

Another key finding was that the most common ‘hub’ of protein connections in both infected and non-infected B. glabrata included leucine aminopeptidae 2 (also known as LAP2). LAP2 is known to play a vital role in neutransmission, hormone activity, immunity and metabolic process in snails and molluscs. Wang et al found numerous S. mansoni miracidial proteins that interacted with B. glabrata’s LAP2 particularly those belonging to a family of cell adhesion molecules. Another interesting result was that these LAP2 interactions continued after infection by the miracidia and more sporocyst proteins were interacting with LAP2 than miracidia proteins, indicating that sporocysts interfere with B. glabatra physiology more intensely.
LAP-like proteins have been found in other molluscs with other infections and in the digestive gland of the mussel Mytilus edulis LAP2 is positively correlated with whole body protein turnover and therefore is critical in the whole metabolism of the mussel.
There is increased interest in freshwater snails of biomedical importance. Key findings of this study and other studies, such as the B. glabrata genome is putting snails in the spotlight for tackling transmission and finding tools for controlling this neglected tropical disease.

Comments