Many parasites have been shown to alter the behaviour of their hosts in order to increase transmission. These changes in infected host behaviour have long captured the imagination of popular culture (think Invasion of the Body Snatchers) and biologists (see previous BugBitten posts on zombie ants and fearless shrimps). In some cases manipulation on the part of the parasite is clear, however in many instances the role of the host and the parasite are difficult to distinguish. Behaviours we observe in association with infection could be the work of the parasite, an adaptive response by the host, a simple by-product of infection, or a combination of all of these.
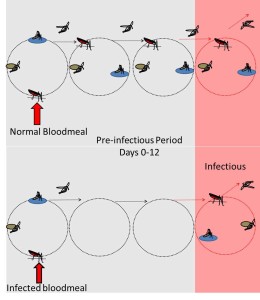 Several studies over the last 30 years have now shown that infection with malaria parasites alters mosquito feeding behaviour. Intriguingly, these changes in behaviour correspond with the different stages of malaria infection inside the mosquito. Generally, feeding behaviour seems to be down regulated during the period that parasites are growing in the mosquito midgut (a period that can last up to 2 weeks) and then up regulated once the parasites have reached the infectious stage (many studies reviewed here).
Several studies over the last 30 years have now shown that infection with malaria parasites alters mosquito feeding behaviour. Intriguingly, these changes in behaviour correspond with the different stages of malaria infection inside the mosquito. Generally, feeding behaviour seems to be down regulated during the period that parasites are growing in the mosquito midgut (a period that can last up to 2 weeks) and then up regulated once the parasites have reached the infectious stage (many studies reviewed here).
These infection-related changes in behaviour are predicted to increase malaria transmission in two ways. First, by decreasing mosquito death during development (females that don’t bite hosts can’t get smacked by them and don’t bear the costs of reproduction) and second, by increasing the likelihood that a female carrying infectious parasites will feed.
Given that other parasites manipulate their hosts and changes in the behaviour of malaria infected females are predicted to increase transmission, it has been assumed that malaria parasites actively control mosquito behaviour.
Host or parasite in charge?
It is easy to fall into the trap of thinking of changes in host behaviour during infection as a simple question of who is in charge, host or parasite. The sloppy term I often catch myself using is, “Who is driving the bus?”. I was corrected by expert in parasite manipulation Shelley Adamo. She encouraged me to think of these changes in behaviour as the result of a “dance” between host and parasite. As new techniques allow us to uncover the mechanisms behind parasite manipulation, we repeatedly see the evidence of complex trade-offs and interactions between parasites and their hosts. Previous work on the effect of malaria infection on mosquito fecundity, much of which were conducted by BugBitten Editor Hilary Hurd, has laid the framework for probing these intricate interactions.
More recent work has certainly complicated this “manipulation story” in malaria mosquitoes. While conducting experiments on the effect of malaria infection on host-seeking behaviour in the Thomas/Read group, we noticed something odd. We observed the previously reported pattern of down and up regulation in host-seeking in Anopheles stephensi mosquitoes infected with a rodent malaria. However, when we dissected our infected group we found that some of these females were not successfully infected. This group of mosquitoes which had been exposed to parasites, but not infected behaved the same way as mosquitoes with parasites inside of them.
If parasites were actively causing changes in host behaviour, how were they doing it without being present? We had “manipulation without the parasite”.
The role of immune challenge
This lead to more experiments demonstrating the mosquito’s own immune response seemed to be involved in regulating these changes in host-seeking behaviour. We used heat-killed E. coli bacteria to stimulate the mosquito immune response. By working with dead bacteria, instead of live parasites, we were able to further investigate how the mosquito immune response might alter behaviour separate from any active manipulation on the part of the parasite.
Similar work was conducted by Ahmed and Hurd in 2006 to unravel the relative contribution of parasite and host to apoptosis in the ovary and reduced fecundity following infection. Surprisingly, not only does this challenge of dead bacteria alter mosquito host-seeking behaviour many days after the challenge (up to 16 days!), it also creates the same pattern as we observe when malaria parasites infect the mosquito.
Insulin signalling patterns
With collaborators in Luckhart group, we most recently reported a potential mechanism for explaining how immune responses triggered in the mosquito midgut might alter host-seeking behaviour. When females were challenged with heat-killed E. coli we observed changes in insulin signalling over a similar timing that we had observed changes in behaviour. These changes in insulin signalling were functionally linked to changes in mosquito feeding behaviour. Excitingly, these same insulin signalling patterns were also observed when we challenged mosquitoes with human malaria parasites. So are malaria parasites manipulating mosquito insulin signalling to increase transmission?
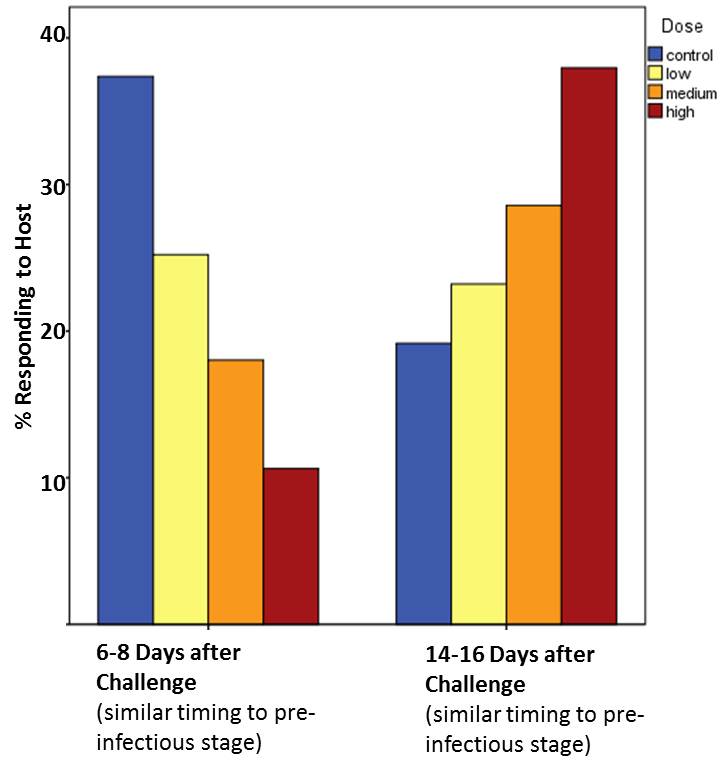
Maybe. The story continues to grow. We now also know that heat-killed E. coli is only able to do this trick when it is delivered to the mosquito in close association with a bloodmeal. We do not observe behavioural alteration with immune challenge alone or after the bloodmeal. This suggests that changes in behaviour we associate with malaria infection depend on an interaction between blood feeding and immune challenge.
Further, we see that these changes are dose specific. The bigger the immune challenge the more dramatic the changes in behaviour. This second result suggests that changes in mosquito host-seeking behaviour associated with malaria infection may be the product of trade-offs or physiological constraints imposed on mosquitoes when infectious parasites in the bloodmeal trigger the immune response.
While we are just beginning to uncover the dance between malaria parasites and their vectors it seems that no matter who is leading, the steps are more complex than we first believed. Also, if there is any subset of mosquitoes which we must understand, it is this small group of infected females which are responsible for transmission. With these changes in behaviour specific to this key group and potentially dramatically increasing transmission, it appears a routine worth learning.

Comments