Tuberculosis (TB) is a zoonotic infectious disease and is still of significance in both humans and animals worldwide. It remains an epidemic in much of the world, causing the death of nearly one-and-a-half-million people each year, mostly in developing countries.
Human tuberculosis is primarily caused by Mycobacterium tuberculosis, while the bacteria M. bovis and M. caprae are known to be the causative agents of bovine tuberculosis (bTB). The three pathogens belong to the Mycobacterium-tuberculosis-complex (MTC) and can cause tuberculosis not only in humans and cattle, but also in other mammalian species.
Bovine tuberculosis in cattle was widespread in Germany until the second half of the 20th century. Due to the effective control and eradication campaigns Germany was declared “officially free of tuberculosis” (OTF) by the European Commission in 1996. Long-term eradication programs have shown that pasteurization of milk in developed countries also reduced the human M.-bovis- and M.-caprae- infections.
Since 2008 cases of bovine tuberculosis in cattle and red deer have occurred consistently in the Bavarian Alps. The Bavarian alpine region is an area with plenty of cattle summer pastures. Cattle on these summer pastures can acquire the infection from red deer and this ruminant is considered to be a reservoir host for bTB.
Bovine tuberculosis can manifest as an acute or chronic infectious disease with a long subclinical phase, in which infected animals do not show clinical signs and with the potential of reactivation after years. After infection, the pathogens hide and persist inside macrophages and usually the host immune system forms a tuberculoid granuloma to prevent further spread of these pathogens (Figure 1). Bacilli within the center of the lesion do not multiply but may remain dormant and the resulting latent infection may persist for years. Immunosuppression can disturb the balance between host control and agent dissemination, allowing replication of the silenced pathogen with reactivation and spread of the infection. However, in animals generalized manifestation of the infection is less common than in humans.
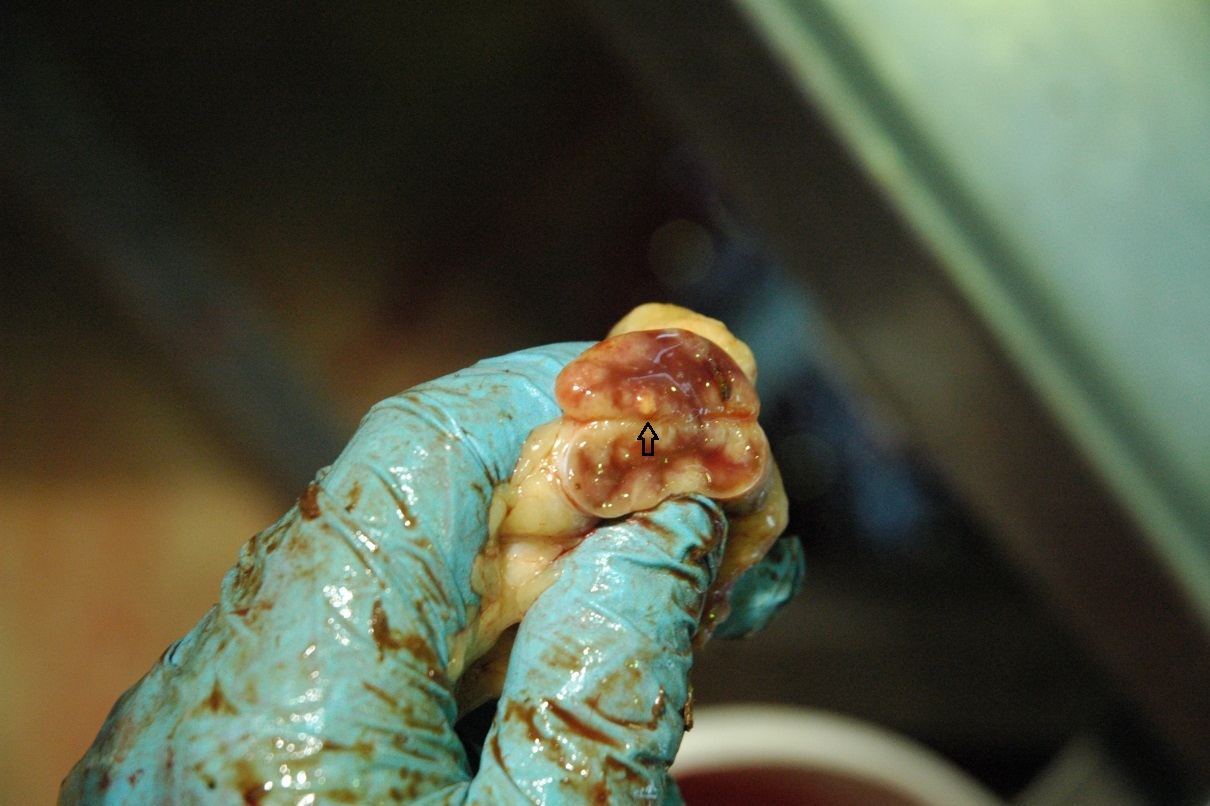
The diagnosis of bTB is challenging. Non-visible lesions may develop in individuals after infection with bTB, which is in contrast to the typical granuloma formation found mostly in lymphoid tissue. Mycobacterial infections are sometimes only very small granulomas or tiny lesions, especially in early stages of infection, which are difficult to detect
As a consequence, diagnostic procedures for the detection of bTB in cattle are challenging, expensive and currently are based on an authority regulated prescribed protocol. Indicating the infectious status of a living animal, cellular immune reactions are measured by using the tuberculin skin test or an interferon gamma release assay. The immune response should then be confirmed after the animals´ death by direct detection of the pathogen via bacteriological culture or its DNA using PCR.
For the extraction of DNA that serves as a template for subsequent PCR, only very small amounts of the tissue of the size of a rice grain are commonly used. This may lead to false negative results due to the inhomogeneous distribution of mycobacteria in tissue material. In addition, animals infected in an early pre-granuloma forming status do not show granulomas at all (non-visible lesions).
This situation makes it impossible to recognize mycobacterial presence and localization in the organ to allow targeted DNA extraction – choosing adequate, bacteria containing tissue sample is like looking for the needle in a haystack.
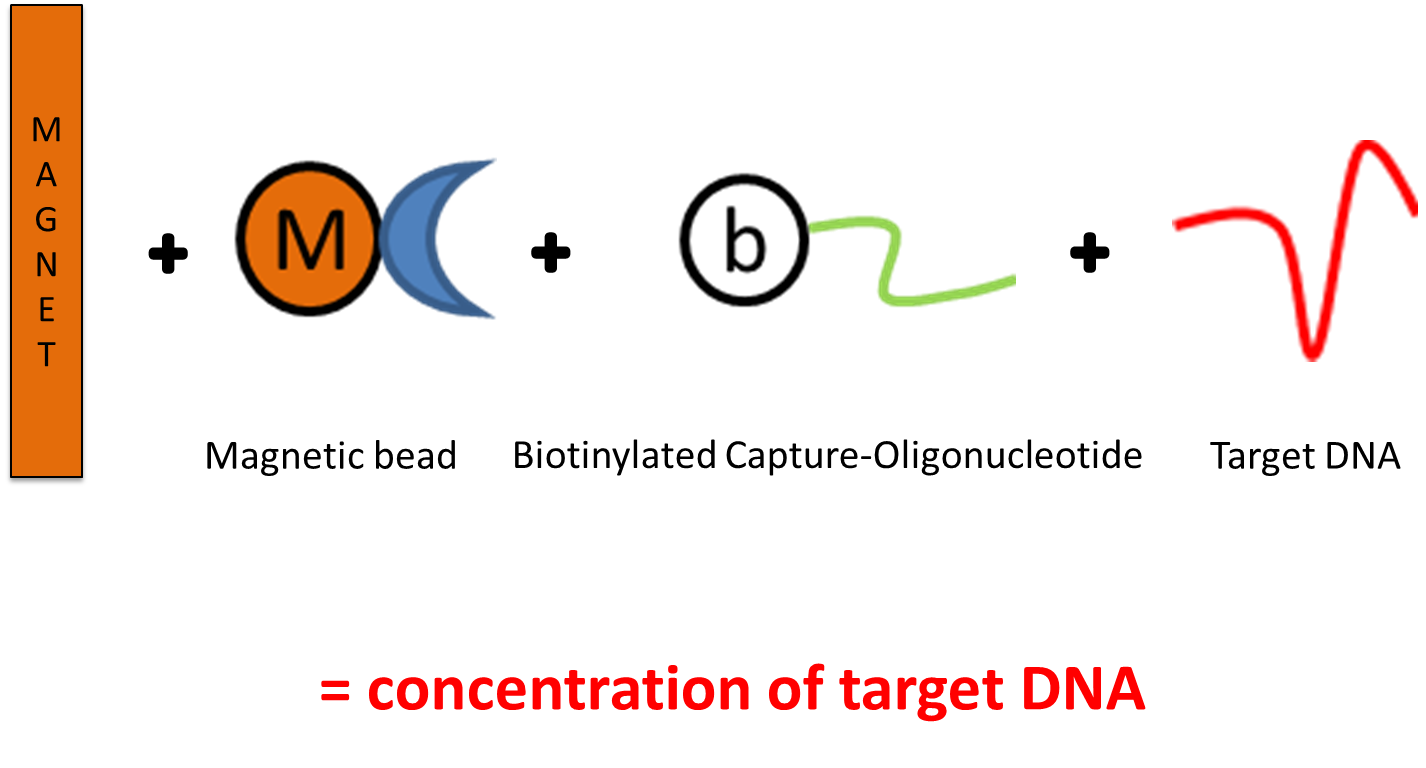
In our study we therefore used a magnet, throwing it into the haystack to find needles (= MTC-DNA). We processed very large sample volumes to increase the probability to include mycobacterial DNA in the investigated sample and used the principle of magnetic capture to concentrate MTC-DNA out of the large sample pool (Figure 2). In a second approach, remaining sediments from the magnetic capture protocol were further processed with a less complex DNA extraction protocol that can be used in daily routine diagnostics.
The use of large sample volumes (up to 15 grams) produced an increase of sensitivity, when our newly developed MTC-DNA detection was applied to animal tissue samples. Both new methods yielded increased detection rates when we analyzed 100 tissue samples from 34 cattle and 18 red deer and turned out to be superior compared to the protocol prescribed by German authorities.
References:
- Fell et al., 2016; Two alternative DNA extraction methods to improve the detection of Mycobacterium-tuberculosis-complex members in cattle and red deer tissue samples (in BMC Microbiology)
- Rettinger et al., 2015: The Region of Difference Four is a Robust Genetic Marker for Subtyping Mycobacterium caprae Isolates and is Linked to Spatial Distribution of Three Subtypes (in Transboundary and Emerging Diseases)
- Broeckl et al., 2017: Investigation of intra-herd spread of Mycobacterium caprae in cattle by generation and use of a whole-genome sequence (in Veterinary Research Communications)
Comments