Advising health systems research synthesis: a Q&A

A Commentary published today in Systematic Reviews describes the rationale of an Advisory Group assembled by The Alliance for Health Policy and Systems… Read more »

A Commentary published today in Systematic Reviews describes the rationale of an Advisory Group assembled by The Alliance for Health Policy and Systems… Read more »

Following the recent publication of the methods for user involvement in a Cochrane systematic review update in Systematic Reviews, we hear from the authors,… Read more »

In our second installment to celebrate International Clinical Trials Day 2015, we hear from a European pharmaceutical company and patients involved in research… Read more »

To celebrate International Clinical Trials Day 2015, BioMed Central asked a European pharmaceutical company, a funder from the United Kingdom, an Ethiopian… Read more »

Last month marked the highly anticipated launch of our new journal, Pilot and Feasibility Studies, which will address the void left by publication bias against… Read more »

New research published today in Trials explored the first-hand experiences that trialists face when conducting and reporting clinical trials. What did they say… Read more »
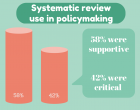
“There is no such thing as public opinion. There is only published opinion” – Sir Winston Churchill Should systematic reviews be used to inform… Read more »

“The registration of all interventional trials is a scientific, ethical and moral responsibility” – World Health Organization (WHO) New research published… Read more »

On 18 September 2014, following a call for comments from the Health Research Authority (HRA) on their proposals to promote transparency in research, key… Read more »

Earlier this week, the European Medicines Agency (EMA) released a draft policy update on access to the data from the EudraVigilance database, which seems to… Read more »