
The ISCB annual conference is designed to promote and enhance the exchange of ideas in the field of clinical biostatistics among biostatisticians, epidemiologists and medical researchers. The majority of the talks were not for the fainthearted as they detailed complex statistical methods with some speakers apologetic if their talk did not contain enough mathematical formulae. The aims for this type of research are clear though, to improve the way that medical research is carried out; a vital task as more and more treatments and interventions are developed and tested.
‘Big data’ in medical research
Several of the sessions and the first plenary talk were on the subject of ‘big data’ in healthcare. The term big data simply refers to the presence of a large volume of data and there is no doubt that this is a modern phenomenon with so many aspects of our lives now being monitored. This can certainly cause alarm but it also provides new opportunities to design more intelligent healthcare that can be tailored to the individual.
Professor Diego Kuonen of the University of Geneva spoke about some of these issues during the first of two plenary talks. He provided a ‘Big tent’ overview of big data and data science in pharmaceutical development during which he warned that we must consider the quality or veracity of the data being used for research very carefully. So much data is now being collected, both from primary and secondary sources, that researchers can run into problems when there is not enough transparency surrounding the collected data. On a more positive note, he hailed this as a new golden era for statistics with the explosion of big data increasing the demand for experts in statistical analysis.
The statistics of sex
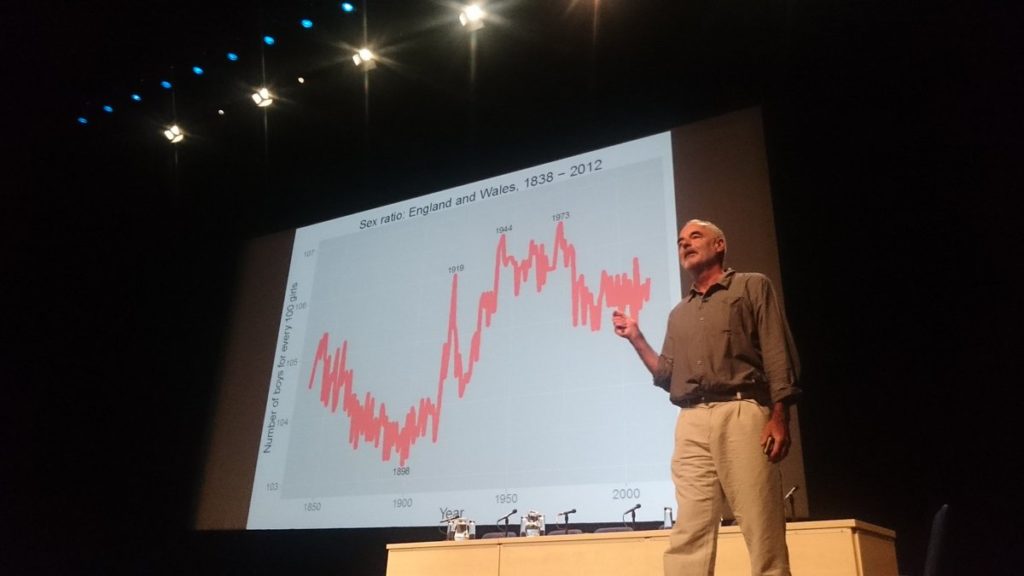
The second plenary talk was a fascinating overview of the statistics of sex by Sir David Spiegelhalter of Cambridge University. Arguably the most accessible talk at the conference, Dr Spiegelhalter led the audience through a series of intriguing statistical observations on human sexual behaviour. He started out with a warning that the reliability of statistics must always be taken into account and as such he has developed a star rating for the classification of statistics which helps him to decide if what’s being presented is reliable.
He also highlighted what can be learnt when we graph seemingly unrelated factors and start to look for patterns. For example, by measuring birth rates of both sexes it was noted that there is a spike in the number of boys born compared to girls at the end of major wars, including World War I and II. Whilst several theories exist about why this might occur, Dr Spiegelhalter’s favoured theory is that the reunion of couples when the men returned from war resulted in increased levels of conception during the early phase of ovulation which is thought to very slightly favour the development of a male foetus.
Developments in the era of personalised medicine
There were also a number of sessions at the conference discussing personalised medicine and how we might adapt clinical trials to cater for differences between patients. The concept of personalised medicine, whereby patients receive a treatment plan tailored to their specific biology, is an exciting prospect in medicine. In stratified clinical trials, differences between patients are measured as the presence or absence of specific biomarkers which could include various molecules or genes related to the intervention being tested. As our knowledge on heterogeneity between patients increases, so too does the complexity of the trials being conducted and so it has become necessary to look for new ways to improve clinical trial efficiency.
Matthew Sydes of University College London spoke on this topic and the development of so called ‘trial platforms’ whereby multiple sub-trials are carried out at the same time. This is hoped to be a more efficient and cost-effective way of carrying out large scale clinical trials as patients are assessed and assigned to the appropriate group or arm in a single screening process. This method also allows the inclusion of additional treatment groups if a new intervention becomes available during a trial. It is clear that flexibility and adaptability in clinical trials is essential in improving their efficacy.
Challenges with this type of trial design are also being uncovered as they are increasingly used. Andrea Jorgensen of the University of Liverpool spoke about the need to improve the reporting of clinical trials to allow them to be used in meta-analyses where articles in the literature are systematically searched and combined in order to get an overall idea of the effectiveness of an intervention. This is an important step when designing a clinical trial as it provides an evidence base to inform researchers. Improvements in the way that meta-analyses are carried out should also allow important findings in the literature to be translated more quickly into treatment plans for patients.
Overall, ISCB 2016 provided a lively and exciting platform for the discussion of many subjects within the field of biostatistics. One of the major take home messages was that the need for accurate and reliable statistical analysis is as important as ever with the increasing complexity of medical research.
2 Comments