
In the course of evolution, arthropods have adapted to an impressive diversity of habitats ranging from lofty mountains to the deep sea. They have, thus, become the most successful group of animals, exhibiting over a million species whose multifaceted external beauty has inspired naturalists for centuries.
The arthropod brain, which has to process a broad range of optic, chemosensory, or mechanosensory input, also shows extraordinary complexity. Throughout the past thirty years, considerable progress has been made in neuroanatomical methods and computer technology. Fuelled by a renewed interest in the evolution of arthropods, these improvements led to new insights into groups that had been neglected for too long.

In Malacostraca—the largest group of crustaceans—familiar subgroups such as crabs, lobsters, crayfish, shrimp (Decapoda), krill (Euphausiacea), or woodlice (Isopoda) have been studied from the neuroanatomical perspective. Only recently have we seen a well-founded reconstruction of the brain in the ancestor of Malacostraca—an important step. But in order to understand how the brain evolved within Malacostraca, it is indispensable to consider also the smaller and less familiar subgroups which dwell in the relict habitats of our planet.
The three blind species, whose brain we studied here for the first time, belong to the malacostracan subgroups of Mictocarididae, Spelaeogriphacea, and Thermosbaenacea. These three groups have in common that all their representatives have lost their eyes long ago—only rudimentary eye stalks, which formerly bore the eyes, may still be present.

These creatures live in caves in total darkness, reportedly using their long first and second antennae to detect food whilst walking or swimming. Under these conditions, animals can only be obtained with considerable difficulty.
Luckily in Bermuda, cave diver Prof. Tom Iliffe from Texas A&M University at Galveston was ready to take the risk and bring the species of Mictocaris halope to light.
Equipped with helmet, cave lamp, and collection gear, Dr. Christian Wirkner and Prof. Stefan Richter from the University of Rostock, Germany, obtained Spelaeogriphus lepidops from Table Mountain, South Africa, with the help of members of the South African Speleological Association, and Tethysbaena argentarii from Monte Argentario, Italy, offering a unique opportunity to investigate the neuroanatomy of these fascinating creatures.

In the laboratory, a combination of semi-thin sectioning (Dr. Christian Wirkner, supported by Ms. Rommy Petersohn), immunolabeling and confocal microscopy (Torben Stemme, Tierärztliche Hochschule Hannover, Germany), and, finally, computer-aided 3D-reconstruction and analysis (my part, supported by Mr. Jens Runge) allowed for an unprecedented description of the brain with respect to its general anatomy as well as its substructures, including nerves, tracts, neuronal somata (cell bodies), and neuropils (coordinative centers).
Like in all crustaceans, the brain in the three blind species investigated here is situated in the middle of the head and divided into three major subunits—proto-, deuto-, and tritocerebrum. An early investigation by Bertil Hanström in 1928 into Decapoda revealed that all species—whether seeing or blind—exhibit an optic center in their protocerebrum that is composed of three major neuropils termed the lamina, medulla, and lobula (a smaller fourth neuropil termed the lobula plate has been discovered later on).
In a striking contrast, the optic center in the protocerebrum of S. lepidops is constituted by only a single neuropil, whose orientation toward the tip of the eye stalk is the only hint at a former visual-coordinative function. Optic nerves are absent. A comparable single neuropil is shown by M. halope and T. argentarii. It could represent a rudimentary optic center too, but as it is oriented sideward, away from the eyestalk, we suggested an alternative interpretation, on the basis of which M. halope and T. argentarii would have lost their optic center completely.
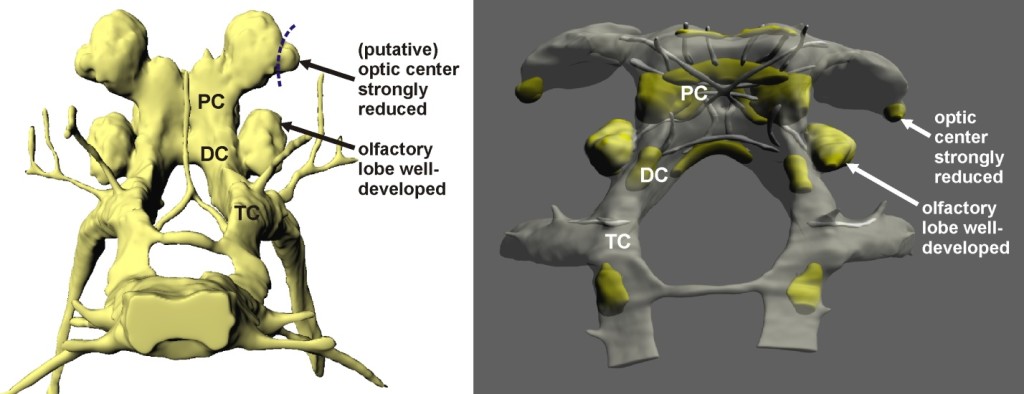
In the species investigated here, the most prominent nerves are connected to the first and second antenna. Both appendages are equipped with numerous mechanosensory hair-like structures, and some additional chemosensory structures have been described at the tip of the first antenna in S. lepidops and M. halope.
In fact, the morphology of the brain implies an important role of the olfactory sense in all the three species. The olfactory lobe in the deutocerebrum of M. halope and S. lepidops is large and organized into spheroidal subunits, corresponding to many other arthropods with a good chemical sense. In T. argentarii, whose olfactory lobe is somewhat smaller, we found a unique accessory neuropil nearby which may also be involved in olfactory-coordinative function.
The three cave-dwelling crustaceans studied here are a vivid evolutionary example of how changing ecological conditions—total darkness—have affected neuroanatomy. The optic neuropils in M. halope, S. lepidops, and T. argentarii were reduced to a degree that is higher than in any malacostracan relative, but comparable to more distantly-related crustacean groups such as horseshoe shrimps (Cephalocarida) or remipedes (Remipedia).
Apparently, the reduction of optic neuropils has occurred several times independently during crustacean evolution. The geographical distribution of Mictocarididae and Spelaeogriphacea reflects the ancient southerly supercontinent of Gondwana, suggesting that the ancestor of these groups lived over 180 million years ago when today’s isolated relict habitats were still situated on one landmass. The crustaceans spent a long time in the darkness to reduce brain parts which have become unnecessary.
One Comment