We know that lowering low-density lipoprotein cholesterol (‘bad cholesterol’) reduces heart attacks and strokes. Yet even when patients have low levels of this bad cholesterol, some still have a heart attack/stroke. We refer to this as ‘residual cardiovascular risk’.
To reduce this residual risk, we need to target other factors. Many have thought that one of these could be high triglycerides (fats) in the blood. People who are overweight or have diabetes often have high triglycerides, usually with a low level of another type of lipid, high-density lipoprotein cholesterol (‘good cholesterol’). This is referred to as atherogenic dyslipidemia.

As a society – not just developed countries but also economically emerging regions– we are becoming fatter, more sedentary, and more likely to develop diabetes. Therefore, high triglycerides are becoming a major issue in the clinic.
So far, doctors have had limited options for tackling high triglycerides. Beyond statins, one treatment is a fibrate, a class of drug first made in the middle of the 20th century. However, in large studies among people already treated with a statin, fibrates did not reduce the residual risk of heart attacks and strokes. The other issue is safety and tolerability. For example, one fibrate interacts with a statin to cause muscle problems so cannot be taken with this first-line treatment.
It’s clear we need new approaches. SPPARMα has evolved in our quest to solve this enigma.
SPPARMα: What is it and how does it act?
To use its full name, a selective peroxisome proliferator-activated receptor alpha modulator (SPPARMα) targets a type of receptor in the body – peroxisome proliferator-activated receptor alpha (PPARα). This receptor is abundant among metabolically active tissues such as the liver, kidney and heart and plays a key role in lowering plasma triglycerides, increasing levels of good cholesterol, and reducing inflammation in the artery wall.
The binding of a drug to the PPARα receptor is complex. The end result is either turning on – or turning off – key genes that control the activity of different metabolic pathways, in particular, those involved in the breakdown of triglycerides so that they can be removed from the bloodstream.
Understanding how PPARα acts set in train thoughts about how to improve on current fibrates. Paracelsus, the man who brought chemistry to medicine, was our mentor in this endeavor.
We know that when a fibrate binds to this receptor it causes a unique pattern of gene activity and related downstream effects. By modifying the structure of this drug, it may be possible to modulate this pattern and so overcome many of the issues encountered with fibrates. In other words, we could make a treatment that is potent and selective for key targets involved in triglyceride metabolism and avoid the side effects associated with fibrate treatment.
Putting the SPPARMα concept into practice
Proposing this SPPARMα concept was the easiest part. Realizing a SPPARMα that we could test in clinical trials was tortuous. Over 1300 compounds were tested before identifying one – pemafibrate (K 877) – as a likely candidate.
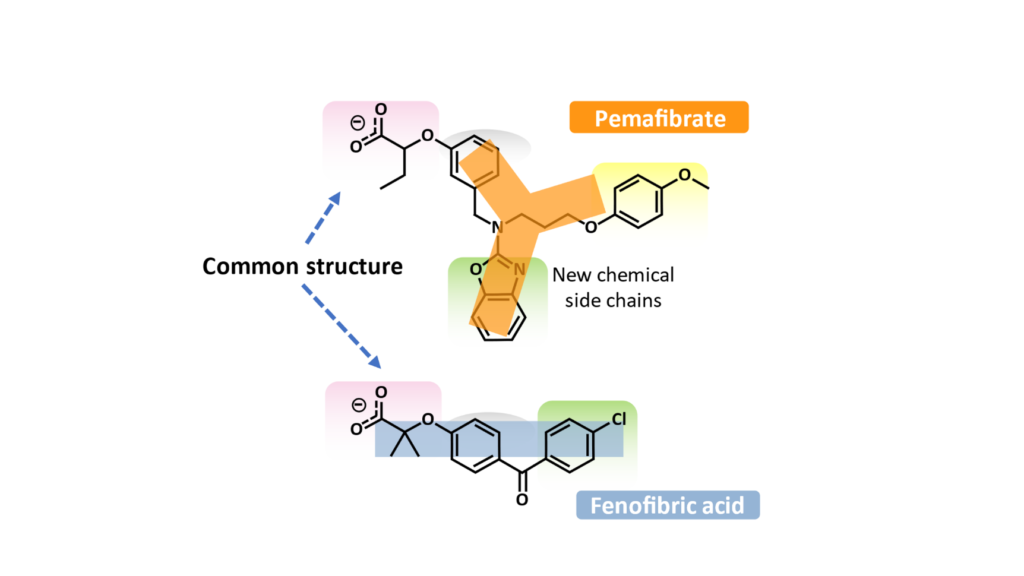
What differentiated pemafibrate from current fibrates was its chemistry. Changing the common structure of a fibrate (such as fenofibric acid, the active drug of fenofibrate) by adding new chemical side chains produced a Y-shaped structure. As a result, pemafibrate was able to bind entirely and more tightly within the ‘business part’ of the receptor. This binding also changed the activity profile of different genes.
Clinical studies have borne out the promise of the SPPARMα concept. Pemafibrate lowered triglycerides, raised levels of ‘good cholesterol’, and also had favorable effects on inflammation, the process responsible for heart disease. Pemafibrate was also well tolerated with no adverse effects on the liver or kidneys. Because of this favorable risk versus benefit profile, it was possible to undertake the final test of the SPPARMα concept.
The critical test for SPPARMα – PROMINENT

One important question remains. Does lowering triglycerides with this novel SPPARMα reduce the residual risk of heart attacks and strokes among patients with well-controlled levels of bad cholesterol? PROMINENT, a landmark study in about 10,000 high-risk type 2 diabetes patients with high triglycerides despite well-controlled levels of bad cholesterol, will be the ‘proof of the pudding’.
Research is a journey. PROMINENT will bring us to the end of one stage in our quest to target residual cardiovascular risk. If proven, SPPARMα offers new potential to manage this risk and reduce the disastrous associated burden of death and disability. This is the key mission of the Residual Risk Reduction Initiative, and all clinicians treating patients at risk of recurrent heart attacks and strokes.
Comments