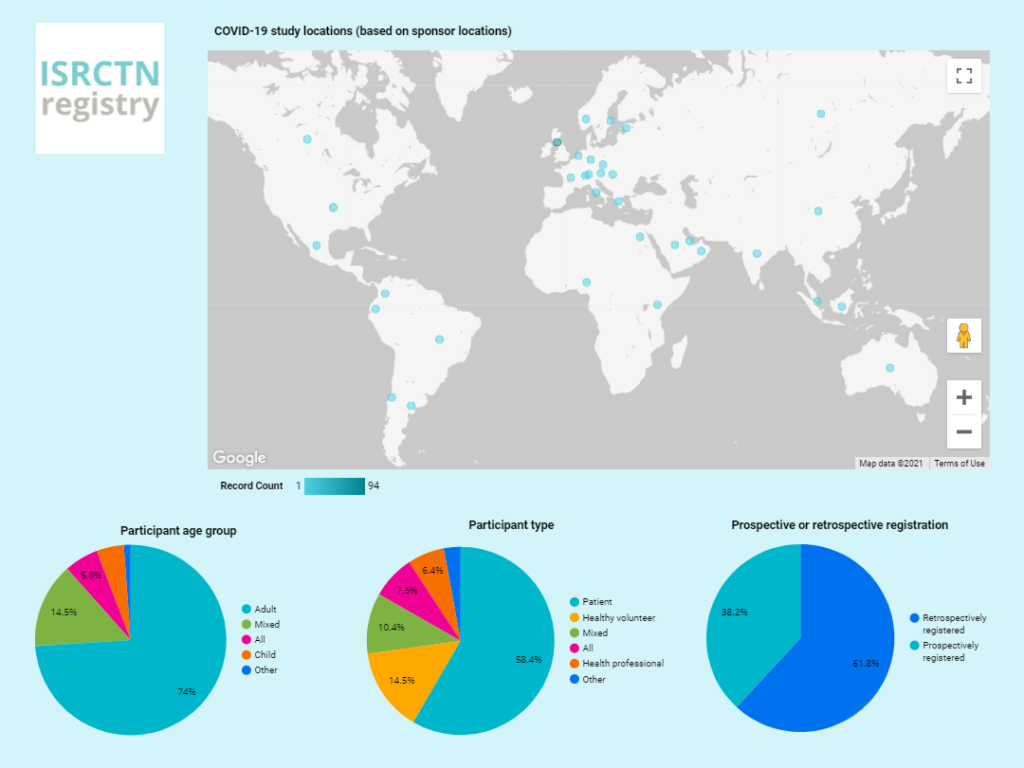
In our blog for Clinical Trials Day 2020 we summarized the COVID-19 studies that had been registered so far at the ISRCTN registry. One year later and there are now over 170 COVID-19 studies registered. In this blog we look back at the results of the platform trials discussed in our first blog and a selection of the repurposed drug treatment trials that have launched since then.
Platform trials
Platform trials compare multiple treatments at the same time using a single protocol, allowing new treatments to be added and ineffective treatments to be dropped throughout the course of the trial.
The World Health Organization (WHO) organized the international SOLIDARITY trial and found that treatment regimens with the repurposed antiviral drugs remdesivir, hydroxychloroquine, lopinavir and interferon had little or no effect on hospitalized patients.
RECOVERY
The UK’s RECOVERY trial is the world’s largest clinical trial of treatments for COVID-19, with more than 30,000 participants across 177 trial sites at the time of writing. It found that the low-cost steroid dexamethasone reduces mortality by up to one third among hospital patients receiving invasive mechanical ventilation or oxygen.
Preliminary results also showed that tocilizumab (a treatment for rheumatoid arthritis) also improves survival and other clinical outcomes. However, the antivirals lopinavir–ritonavir and hydroxychloroquine, the antibiotic azithromycin and convalescent plasma did not reduce mortality. The antiviral antibody cocktail REGN-COV2 and the anti-inflammatory drugs aspirin and colchicine have since been added to RECOVERY.
PRINCIPLE
The PRINCIPLE trial is testing community treatments for suspected COVID-19 in older people and those with pre-existing conditions at GP practices in England. They are looking for treatments to help people with COVID-19 symptoms recover quickly so that they don’t need to go to hospital.
Hydroxychloroquine was originally included in the trial but was discontinued based on the results of the other studies. The antibiotics azithromycin and doxycycline were also found to be not generally effective. PRINCIPLE is currently testing the commonly-used inhaled corticosteroid budesonide and the anti-inflammatory colchicine.
Other platform trials
Other platform trials include ACTT-EU/UK, which found that remdesivir shortened the time to recovery in hospitalized adults. ACCORD-2 is testing treatments including bemcentinib, MEDI-3506, acalabrutinib and zilucoplan.
Our current knowledge of severe COVID-19 suggests that the immune system is overactivated in response to the infection, which may lead to organ damage.
Our current knowledge of severe COVID-19 suggests that the immune system is overactivated in response to the infection, which may lead to organ damage. Medications licensed for patients with autoimmune diseases such as rheumatoid arthritis and unlicensed medicines in trials for these conditions are being tested to see if they can prevent this overactivation of the immune response.
The platform trial REMAP-CAP found that immune modulation with tocilizumab and sarilumab improved critically ill patients’ outcomes including survival, while CATALYST is comparing treatments including the monoclonal antibodies gemtuzumab ozogamicin, namilumab and infliximab to see if they can reduce inflammation in the body caused by the virus.
New trials
Immune modulation
Other monoclonal antibodies being tested include adalimumab, which is being investigated in the AVID-CC trial to see whether giving it to patients outside hospital can prevent progression to respiratory failure or death.
TACTIC-R is also recruiting patients at an early stage when they are starting to show mild lung complications to see whether the repurposed drugs ravulizumab and baricitinib can prevent organ damage and reduce the need for ICU treatment and ventilated breathing support.
Otilimab is being tested in the OSCAR trial specifically in patients at a later stage who have developed severe lung complications and require oxygen support or mechanical ventilation.
Antivirals
Several other antiviral drugs are in trials including favipiravir – the GETAFIX study is looking at whether giving it to patients with milder COVID-19 symptoms helps with their symptoms and reduces the time it takes to recover.
The IONIC study is testing whether a combination of the antiviral oseltamivir and a new medication (IMU-838) can improve the time it takes to recover from COVID-19. SNG001, an inhaled drug that contains the antiviral protein interferon-β, is being tested in the SPRINTER trial to see whether it can accelerate the recovery of hospitalized patients receiving oxygen.
Other repurposed drugs
Other drugs in trials include almitrine, which is being tested to see if it can improve blood oxygen levels and reduce the need for oxygen therapy and other forms of breathing support. The RECOVER trial is investigating whether the blood pressure-lowering drug losartan can improve patient outcomes.
The ILIAD-7-UK trial aims to find out whether the drug CYT107 can reduce mortality by reversing lymphopenia (where patients have abnormally low levels of white blood cells called lymphocytes) and T-cell exhaustion (which prevents the body from dealing with chronic viral infections).
The take-home message
The ISRCTN registry continues to support global research on COVID-19 by giving priority to prompt registration and reporting of COVID-19-related studies. All registered studies are made available worldwide through the WHO’s International Clinical Trials Registry Platform (ICTRP), and all UK studies appear on Be Part of Research.
In the second part of this blog we discuss the vaccine trials and studies looking at testing and the impact of COVID-19 on other conditions. To find out more about how BMC are celebrating Clinical Trials Day 2021, please visit our dedicated page.
Comments