This post has been been posted by Larissa Shamseer on behalf of The CONSORT Group
Contributions by: Doug Altman, Ken Schulz, David Moher, Sally Hopewell
2016 marked 20 years since the publication of the first CONSORT Statement. It was the first reporting guideline of what is now a field of 350 and growing, and has been widely regarded as a milestone in health research.
But its developers still ponder how to improve it, widen its uptake and adherence, and increase its impact. Over the years, the focus has primarily been on what journals and authors should do, such as endorsing or adhering to CONSORT, respectively. However, these parties exist within a complex system of research and publication involving multiple stakeholders.
Together with the CONSORT Executive team, we put together a list of a few key stakeholders who might play an increased role in better trial reporting. We recommend who, what, how, when, where, and why things could be done better with regards to transparency in trial reporting. Importantly, we end with the consequences of doing nothing to improve this situation.
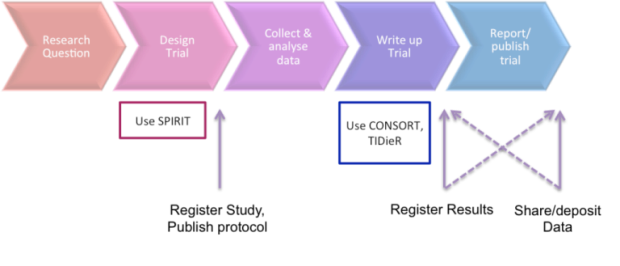
Above is a representation of the basic stages in the study process for trials and where basic reporting requirements and recommendations fit in. The stakeholder guide below shows how SPIRIT (trial protocol recommendations) and CONSORT fit into broader actions towards better trial reporting, such as registering trial protocols and results and sharing trial data.
Key stakeholders
Funders
| What | How | When | Where? | Why? |
| Require that trial funding applications address SPIRIT checklist items for trial protocols | Provide an application submission template/form based on SPIRIT checklist items;
Provide guidance on how to report each item or link to the SPIRIT Explanation and Elaboration. |
Prior to grant submission | Enable completion of protocol template within online grant submission systems, where possible | Improve investigator knowledge of what to include in trial application; prompts investigators to consider details of trial methods and anticipate potential problems |
| Carry out peer review based on SPIRIT items | Provide guidance on how each item should be reported (from SPIRIT Explanation and Elaboration);
Use SPIRIT checklist items as part of grant evaluation criteria |
During grant peer review | In electronic evaluation system or appended to grant peer review decision | Improve consistency of application adjudication; Reduce time to decision |
| Ensure that published reports for all funded trials adhere to all CONSORT checklist items | Withhold partial funding until time of publication; review pre-submission manuscript/report to ensure that it completely reports all CONSORT checklist items | Prior to manuscript submission to journals | Electronic system for submitting and reviewing completed trial reports | To deter selective reporting; Transparently and completely reported grant-funded research will reflect positively on the funding agency |
Academic institutions
| What | How | When | Where? | Why? |
| Endorse SPIRIT and CONSORT in instructions to authors | Write a supportive statement with and expected action by authors regarding protocol or trial reporting | Immediately/ ongoing | Instructions to authors | Poor reporting hinders readers and may prevent inclusion in systematic review; Journal endorsement of CONSORT is associated with better reported trials |
| Check that all SPIRIT or CONSORT checklist items are adhered to (i.e., reported) in published protocols and trial reports | Provide guidance to peer reviewers or use a reviewer assessment template based on SPIRIT or CONSORT items | During peer review | Electronic system for documenting or submitting peer review decisions. | Use of CONSORT to carry out peer review yields more completely reported trials |
| Ensure all journals in a given specialty uniformly endorse CONSORT and SPIRIT | Motivated/leading editors within a specialty should reach out to all other editors across the field to coordinate endorsement and implementation activities | Anytime | By publishing an editorial across journals of the specialty indicating the uniform requirements | Authors will be more compelled to comply with reporting standards if uniform standards exist across a specialty |
Journals
| What | How | When | Where? | Why? |
| Endorse SPIRIT and CONSORT in instructions to authors | Write a supportive statement with and expected action by authors regarding protocol or trial reporting | Immediately/ ongoing | Instructions to authors | Poor reporting hinders readers and may prevent inclusion in systematic review; Journal endorsement of CONSORT is associated with better reported trials |
| Check that all SPIRIT or CONSORT checklist items are adhered to (i.e., reported) in published protocols and trial reports | Provide guidance to peer reviewers or use a reviewer assessment template based on SPIRIT or CONSORT items | During peer review | Electronic system for documenting or submitting peer review decisions. | Use of CONSORT to carry out peer review yields more completely reported trials |
| Ensure all journals in a given specialty uniformly endorse CONSORT and SPIRIT | Motivated/leading editors within a specialty should reach out to all other editors across the field to coordinate endorsement and implementation activities | Anytime | By publishing an editorial across journals of the specialty indicating the uniform requirements | Authors will be more compelled to comply with reporting standards if uniform standards exist across a specialty |
Researchers/Authors
| What | How | When | Where? | Why? |
| Address all SPIRIT checklist items when preparing trial protocols/ proposals | Use SPIRIT items as a writing guide/template | While preparing trial protocol | Electronic trial protocol document | Anticipate problems in the research process, guide research conduct, prevent arbitrary decision-making during conduct; To ensure usability and improve uptake of research by health professionals and other researchers |
| Address all CONSORT checklist items and any relevant CONSORT extensions when preparing a trial manuscript for publication. Use TIDieR for describing interventions in detail. | Use checklist as a writing guide/template | During the writing process | Electronically | May improve chances of getting published; Well-reported trials reflect well on individual researchers |
Failing to act
The human, fiscal, and scientific costs of doing nothing to improve the reporting of trial are serious:
- Poor, incomplete, and selective reporting will prevail
- Poorly and incompletely reported research is unusable by readers (including systematic reviewers and health professionals)
- Patient contributions and time are wasted
- Poorly reported research can’t be replicated in practice
- Funder investments in trials are wasted if they are poorly planned or if findings are unusable
- Poor reporting reflects poorly on researchers, institutes, journals, and funders
This June, a new tool to adjudicate trial reporting is launching, StatReviewer. It provides an automated assessment of trial reporting, feeding back where improvements should be made. It is intended for publishers and journals wanting to gauge completeness of reporting at their journals, but could be used by anyone who has an interest in improving reporting, including authors.
CONSORT Website (CONSORT checklist, flow diagram, extensions, translations): www.consort-statement.org
SPIRIT Website (SPIRIT documents, translations, tools): www.spirit-statement.org
Comments