
Dr Amir Sonnenblick is a Research Fellow at the Breast Cancer Translational Research Laboratory, Institut Jules Bordet, Université libre de Bruxelles.
Could you give a brief introduction to the use of trastuzumab for treating breast cancer, and explain the problems associated with resistance?
HER2 is amplified in about 15-25% of breast cancer patients and is associated with worse outcome. Scientists characterized trastuzumab (Herceptin®), a monoclonal antibody that was reactive to HER2. In 1991 trastuzumab was tested in human subjects and, in 1998, it received FDA approval for the treatment of HER2-positive metastatic breast cancer. Trastuzumab is a remarkably effective therapy, both in the metastatic and adjuvant settings of HER2 positive breast cancer. The adjuvant trials generated highly consistent results showing that the addition of trastuzumab to chemotherapy decreased recurrence by 50% and mortality by 30%. However, not all women with tumors expressing high levels of HER2 respond to trastuzumab.
Why is it important to study molecular markers of resistance?
After failure of trastuzumab, there is a medical need for new pharmacological agents that may reverse trastuzumab resistance and improve the patients’ outcome. Also, identifying patients with trastuzumab resistance in the adjuvant setting may teach us which patients may benefit from alternative drugs or combinations. Several potential mechanisms to explain trastuzumab resistance have been proposed, including loss of PTEN, excessive signaling through the insulin-like growth factor-I receptor, or expression of p95HER2, a truncated form of the HER2 receptor that has kinase activity but lacks the extracellular trastuzumab-binding domain.
… identifying patients with trastuzumab resistance in the adjuvant setting may teach us which patients may benefit from alternative drugs or combinations.
What were the key findings from your research?
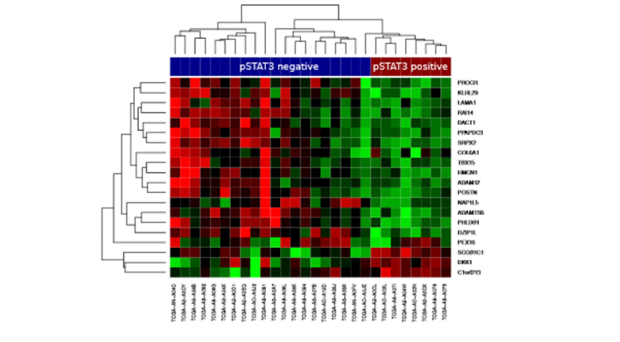
We showed that STAT3 phosphorylation was associated with a distinct gene expression signature in HER2 positive cancers, and that this signature was associated with trastuzumab resistance in clinical samples from a prospective randomized trial. We also found that tumors with activated pSTAT3 were associated with loss of PTEN, elevated IL6, and stromal reactivation.
Were there any particular challenges to overcome in your study?
Our repository contained gene expression data from FFPE tissues. We therefore developed an integrated proteomic-genomic approach that enabled us to use this dataset. We also had to show that similar results were obtained using fresh frozen or FFPE-derived datasets.
What are the implications of the finding that pSTAT3 is associated with trastuzumab resistance?
We propose that the IL6-STAT3-stromal feed-forward loop, which can be enhanced by PTEN loss, is predictive of trastuzumab resistance. Therefore, inhibiting the IL6-STAT3 pathway may be a valuable addition to trastzumab treatment of HER2-positive breast cancer, in an attempt to overcome resistance. In the adjuvant setting IL6/STAT3 markers may identify patients which might need more treatment options.
Are there any agents targeting STAT3 that are currently approved or under clinical development?
There are few IL6-Jak-STAT3 monoclonal antibodies and tyrosine kinase inhibitors in different stages of development. For example, the U.S. Food and Drug Administration (FDA) have approved siltuximab (anti IL6 monoclonal antibody) and tocilizumab (IL-6 recpetor blocking antibody) in multicentric Castleman’s Disease. Tocilizumab as well as JAK inhibitor are also used to treat rheumatoid arthritis. It would be intriguing to evaluate these drugs in the treatment of HER2 positive breast cancers.
How could your findings be extended?
Our next aims are to evaluate in the clinical setting whether one of the anti IL6/ STAT3 agents, combined with HER2-targeted therapy, have effect in HER2-positive breast cancers resistant to standard therapy. We would also like to validate using larger sets whether IL6/pSTAT3 markers could serve as predictive biomarkers for resistance to trastuzumab in the adjuvant setting.
 BMC Medicine: passionate about quality, transparency and clinical impact
BMC Medicine: passionate about quality, transparency and clinical impact
2014 median turnover times: initial decision three days; decision after peer review 41 days
Comments