Unpublished research is a serious problem for evidence-based decision making in healthcare, and this was recently highlighted on BBC Radio 4’s Today programme and in an entire issue of the BMJ. Systematic reviews aim to present the totality of the evidence, and a problem for those preparing and maintaining these reviews is how to find unpublished studies and data. But, even when clinical trials are reported in journals and their supplements the formats and descriptions are widely heterogeneous and studies can remain difficult to discover and challenging to compare with similar trials.
Clearly connecting trial-related publications is a way to help with this problem and is a major goal of BioMed Central’s Threaded Publications initiative. To achieve its fundamental aims of connecting all digital published content relating to the evidence about a particular trial, however, Threaded Publications must go beyond a single journal or publisher. Through our partnership with CrossRef – an organisation founded by publishers, for publishers – and engagement with editors and publishers we hope to achieve interoperability across different publishing platforms. The desired outcome is that articles reporting the protocol or the findings of a trial published in different journals or by different publishers will be linked in a thread, which should also include the trial’s entry in a research register.
The Threaded Publications concept and prototype, which was demonstrated at a number of medical communications and publishing meetings in 2011, builds on CrossRef’s widely-established digital object identifier (DOI) infrastructure and more recently-developed CrossMark tool. CrossMark, which has recently been implemented by the Royal Society, conveys additional information, such as corrections and updates, about journal articles in a standard way. This ‘non-bibliographic article-level metadata’, to use its more technical term, can be displayed for any article which uses CrossMark – including, in principle, the publication thread – by a reader clicking on the CrossMark logo (see figure; but noting that the terms used in the sample thread need further work).
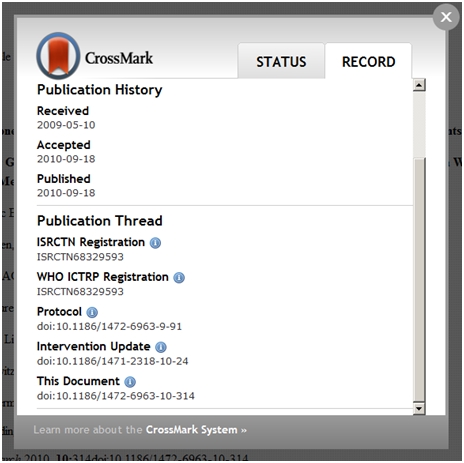
With cross-publisher interoperability comes a need for standardization and, specifically, a simple “ontology” (a controlled vocabulary – a bit like a dictionary – of agreed terms on a specific topic) of different article types that might be included in a thread of publications. This may sound complicated or difficult to implement but, if we know what each element of the thread is, and where it sits in the thread, we stand a good chance of making that information usefully available to a reader, patient, practitioner, or researcher, including those preparing systematic reviews. Agreeing on the terminology for describing the literature will help achieve this.
Any journal or publisher participating in the Threaded Publications scheme will need to agree to and use these standard terms – whether they publish the study protocol, results, methodology, an editorial discussing the trial, the dataset, or something else. Trial registration records, such as those in the ISRCTN register, are central to transparent reporting but these are not currently citable and discoverable in the same way as journal articles. A logical development to help implement Threaded Publications would be assign DOIs to trial registration records. This would better enable citation – and possibly academic credit – of these records in the same fashion as journal articles might potentially provide more motivation to ensure the completeness of the registry entries.
The team at BioMed Central, working closely with our external and recently expanded advisory group (see Acknowledgements below), have drafted a concept document, for standard terms describing publications that might be related to a clinical trial.
Professor Mike Clarke, Director of the All-Ireland Hub for Trials Methodology Research put the current complexity of the literature into context: “A research study is so much more than the few thousand words that make it into a journal article describing its findings and, yet, this is all that is readily available for most studies. By providing the means to thread together a published ‘life story’ for a study, BioMed Central are helping to release its full potential to influence practice and future research. Users will be able to enter the thread at any point and travel in either direction to find out more about the design, conduct and outcome of the study.”
To be part of a threaded publication a document must be one that is explicitly relevant to the published evidence associated with a particular trial. This sets this functionality apart from currently available features that link to [possibly] ‘related articles’. We believe we have captured all the types of article or record that currently exist in the literature and scientific databases, but we may have missed, or have named some inappropriately. We invite the clinical research and publishing communities to download the document and share your comments on our first step towards an ontology of publications related to clinical trials.
Acknowledgements: Thanks to Prof Doug Altman, Sir Iain Chalmers, Prof Mike Clarke and Dr Ben Goldacre for their comments on an earlier draft of the ontology, and this blog.
Footnote: The ontology concept document is licensed under a Creative Commons Attribution License.
Can i call this “EBM Update”. This is brilliant and a bonus to EBM and research todate and in future. Exclusively cool. Thank you for making LITERATURE-SEARCH friendly! God bless.
F1000 would like to suggest a few additions to your proposed ontology:
1. ‘Recommendation’: F1000’s faculty members select and review papers they believe to be of special significance. It would be beneficial to include these F1000 Recommendations in the threads.
2. ‘Guideline’: Not all clinical guidelines are published in journals and given DOIs, but many are and all refer to trials. Good to include them.
3. ‘Meta-analysis’: At a pinch meta-analyses could use the ‘Systematic Review’ tag, but they’re not actually the same thing so we recommend adding ‘Meta-analysis’ to the ontology.