Since its development in the 1970s, the antihelmintic Praziquantel (PZQ) has been the primary treatment for schistosomiasis infection, and mass drug administration (MDA) has been the main form of controlling this devastating disease globally.
These MDA campaigns have significantly reduced the prevalence of schistosomiasis, with a reported ~60% decrease in disease prevalence among school-aged children, considered to be the group most vulnerable to schistosomiasis-associated morbidities.
Despite these successes, considerable challenges remain to be overcome if we are to achieve the ambitious goal of eliminating schistosomiasis as a public health problem in all endemic countries and interruption of transmission in certain regions by 2030, as outlined by the World Health Organisation.
Such challenges include (but are not limtied to) the persistence of infections despite strenuous control, the occurrence of infections in new regions due to human and animal migration, and re-emergence of infections in regions considered to have been successfully controlled.
As PZQ monotherapy is heavily relied on for control of schistosomiasis and has been intensively and widely applied, the possibility that parasites evolve resistance to praziquantel is a major concern. Recent discovery of the drug’s molecular target, the parasite’s transient receptor potential melastatin ion channel (TRPMPZQ), has allowed for better tracking of resistance-related mutations.
While laboratory studies have shown that PZQ resistance can develop rapidly, resistance has only been detected in very limited cases in schistosomiasis endemic countries. As resistance to PZQ (or even reduced susceptibility) would seriously impede our ability to fight schistosomiasis worldwide, more widespread genomic surveillance of variation and identification of resistance-conferring TRPMPZQ alleles is of great importance.
In a recent (and soon to be published) preprint, Berger et al undertook the most comprehensive investigation of TRPMPZQ variation to date by sequencing and assembling 570 Schistosoma mansoni genomes from across 8 endemic countries. The research focused on parasite miracidia collected in two countries (Tanzania and Uganda) that remain highly prevalent for schistosomiasis despite decades of MDA, where they collected parasites before and after a PZQ MDA campaign.
In doing so, not only could the researchers identify the prevalence of possible resistance-conferring mutations in the TRPMPZQ gene among these sampled parasites, but could also gain valuable insight into schistosome evolution and population genetics under intensive MDA-based control.
Schistosoma mansoni genetic diversity, TRPMPZQ variation, and Praziquantel resistance
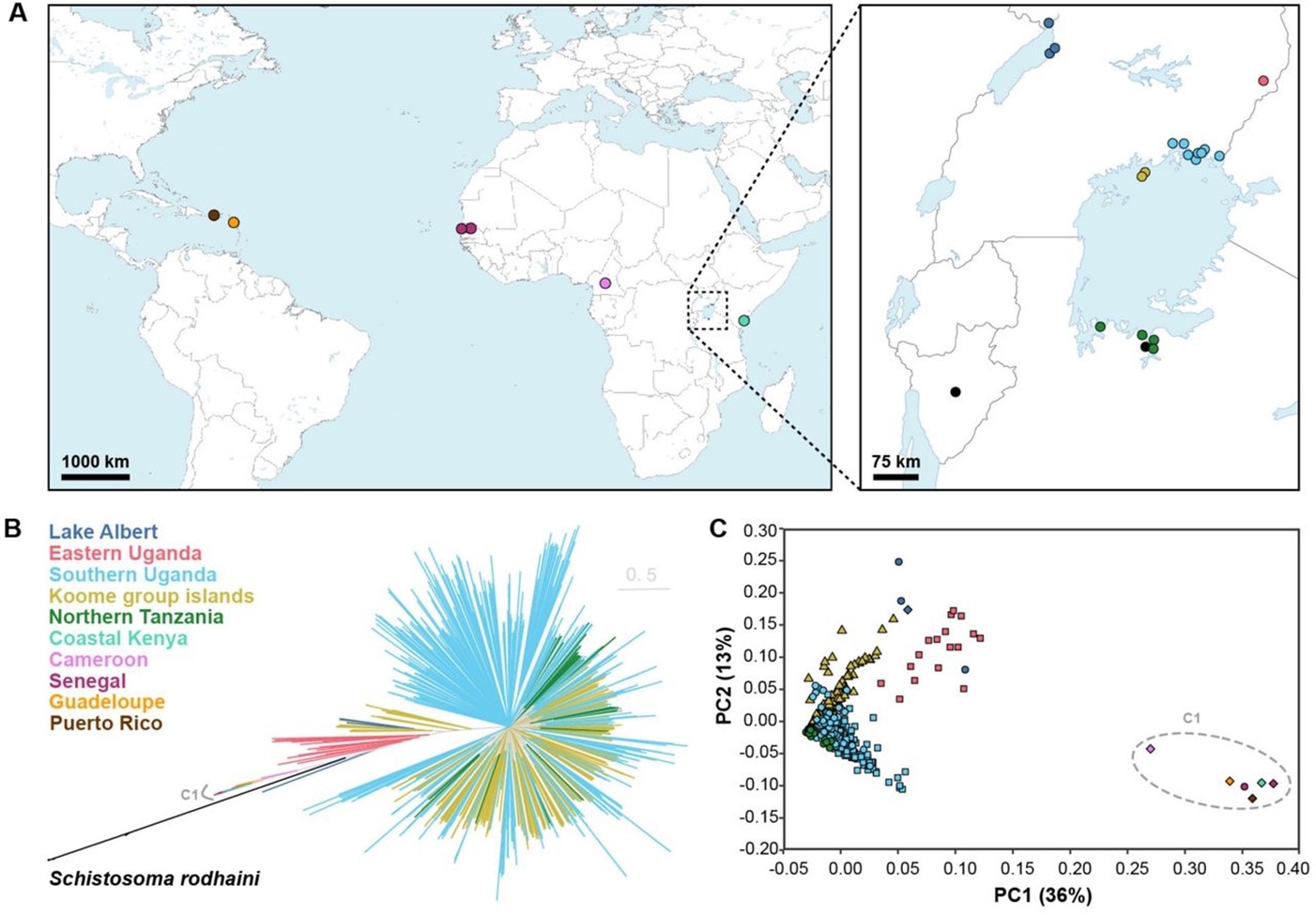
By mapping and assembling the sequence data from these 570 S. mansoni parasites against the reference genome, over 35 million single-nucleotide polymorphisms (SNPs) and 6.6 million INDELs were identified.
By assessing the degree to which these mutations are shared among the sequenced parasites, they found that parasites grouped into distinct clusters based on the region they were sampled, such as those from East Africa, West Africa, and the Caribbean. However, within these regions low levels of differentiation were found, especially between Lake Victoria and Lake Albert.
Looking at TRPMPZQ specifically, these SNPs were found to be distributed across much of the gene and were highly variable among the sampled parasites. SNPs were characterised based on whether they resulted in an amino acid change in the parasite’s TRPMPZQ protein channel, where 496 amino acid changes were identified which may impact the structure and functionality of the channel.
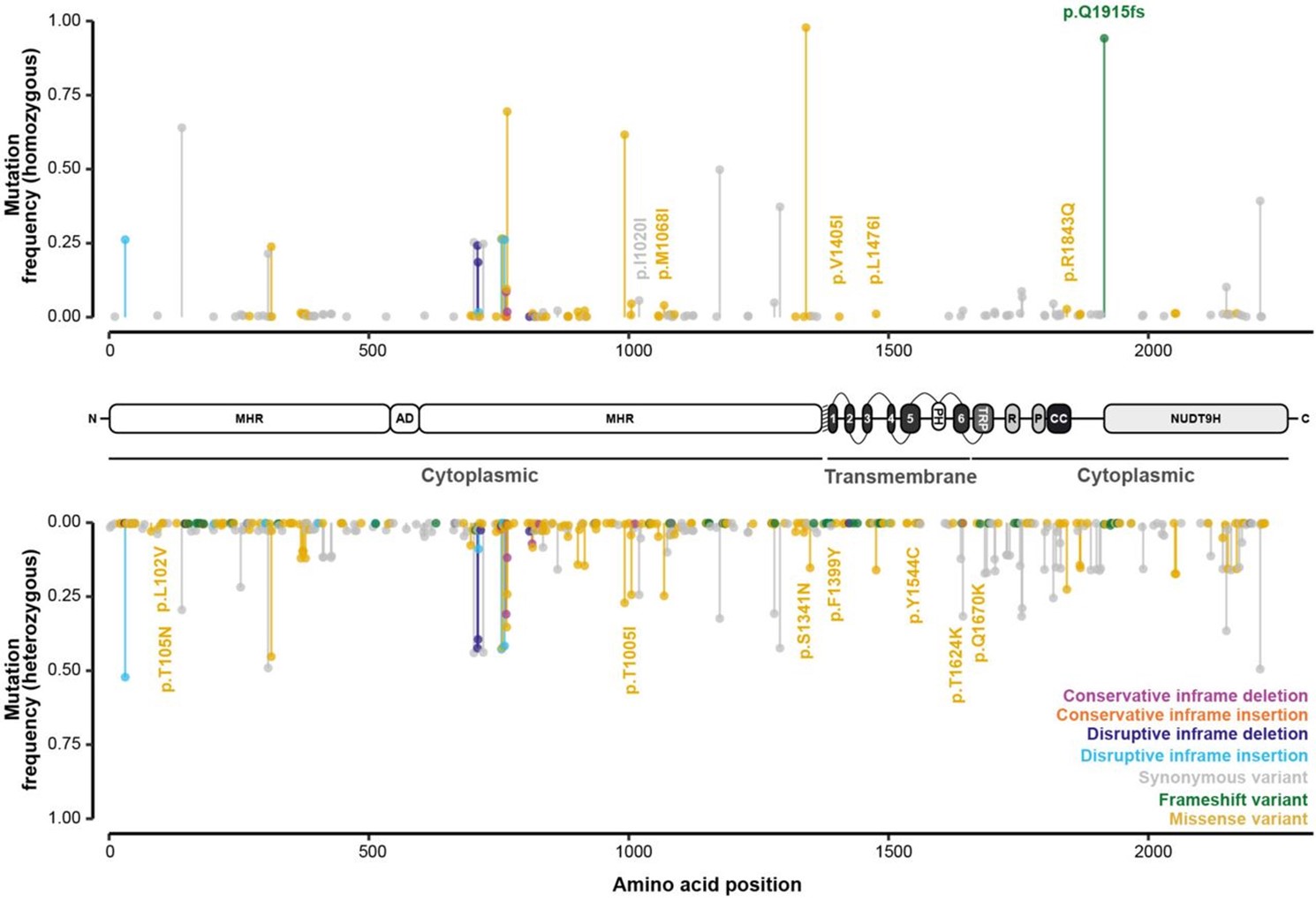
The researchers then generated point mutations (mutagenesis) at 12 of these key amino acids using live parasites in the lab (in vivo), and observed their impact on TRPMPZQ channel sensitivity to PZQ. Eight of these had no affect on PZQ sensitivity, while 2 mutations (p.T1624K and p.R1843Q) conferred reduced sensitivity, and two others (p.Y1554C and p.Q1670K) caused a complete loss of TRPMPZQ channel sensitivity to PZQ.
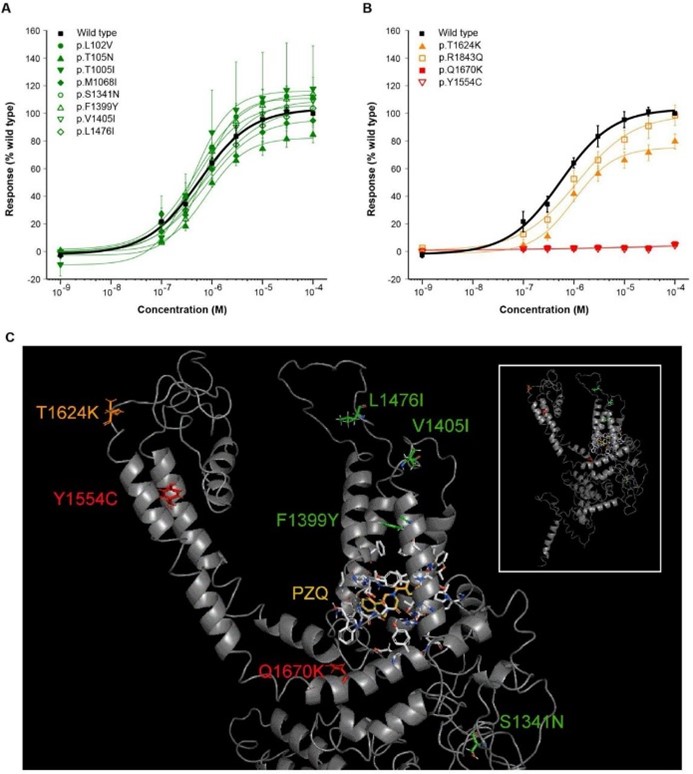
Protein modelling of the TRPMPZQ channel showed these amino acid changes were present in regions critical to TRPMPZQ function, such as p.Q1670K within the intracellular TRP helix that might interfere with the proper regulation of the channel’s activity.
When looking for the presence of these mutations in parasites before and after PZQ treatment, some of these parasites were found to have survived treatment and certain mutations were found more frequently in post-treatment populations, possibly indicating a link to ‘failure’ of PZQ treatment in these individuals.
Concluding remarks
As MDA efforts for schistosomiasis continue, and likely increase in order to reach elimination targets, so does the risk that praziquantel resistance emerges in endemic populations. As a result, studies such as this one characterising the genetic diversity of schistosomes and variation in drug targets possibly conferring resistance/reduced susceptibility become increasingly essential.
Although some resistance-associated mutations were present in the parasite populations, no high-frequency variants associated with resistance were identified. This is somewhat reassuring given the long-term use of MDA interventions, but we must remain vigilant.

Comments