
Toxoplasma gondii is a protozoan parasite that can infect almost all endothermic animals, including humans. As it can only undergo sexual reproduction in members of the cat family, other mammals and birds act as intermediate hosts.
An infected cat sheds long-lived oocysts in its faeces. If these cysts are ingested by an intermediate host the cyst wall is dissolved and the parasites invade the epithelial cells lining the intestine, where they differentiate into a motile, rapidly multiplying stage. Intestinal infection causes inflammation and alteration of the gut microbiota.
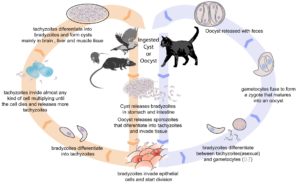
A breach in the intestinal barrier occurs and parasites are released to travel throughout the body. In response to the host immune reaction, these motile forms convert to a semi-dormant stage (a cyst), inside tissue cells, particularly in the brain, eyes and skeletal muscles.
Toxoplasma infection in humans
The parasite occurs worldwide with, in some countries, over 50% of the population infected.
Although most people are asymptomatic, complex reactions to infection occur. The parasite breaches the blood-brain barrier and induces neuronal apoptosis, which may be the mechanism responsible for the neurological disorders associated with toxoplasmosis.
Intestinal inflammation and the production of cytokines by innate immune cells are crucial in controlling T. gondii infection by inhibiting parasite reproduction. But this can impact the gut microbiota which, in turn may affect interactions between the gut microbiome and the brain.
Researchers from Yunnan Agricultural University, in China, have shown that a metabolite from gut bacteria mitigates the intestinal inflammation associated with T. gondii infection by alleviating the loss of some gut bacteria. Further research into the role of gut bacteria in pathology caused by T. gondii has been reported in a paper recently published by a team from the Southern Medical University, People’s Republic of China, and is summarised below.
What did they do?
Experiments were conducted to compare the effect of T. gondii infection on gut microbiota and the cognitive behaviour of mice with a functional immune system and with immunocompromised mice. The former were pathogen-free wild type (WT) mice and the latter vimentin gene knockout mice (vim−/−). Mice were orally infected with a low or high dose of T. gondii cysts or used as uninfected controls.
Behavioural tests
The mice were given a variety of standard tests three months post-infection. These involved swimming tests, maze negotiation and open arena tests to assess spatial negotiation, memory, anxiety and sociability.
Results from the behavioural assays suggested that infection produced a decline in learning and spatial memory abilities and induced depression-like symptoms. These decreases were significantly greater in the WT mice. In addition, anxiety levels were significantly reduced among T. gondii-infected mice, and more so amongst the WT mice than the immunosuppressed ones.
In the brain
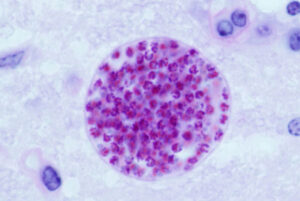
Brains were then examined for T. gondii cyst formation, which was significantly higher in the WT mice compared with the immunocompromised vim−/− mice.
Transcriptome sequencing was performed on brains from mice with chronic infection. Disparities were found in transcriptome levels, particularly in apoptosis-related genes which were significantly up-regulated in WT mice. This was confirmed by immunofluorescence staining of brain tissue.
This finding led the researchers to determine whether the previously reported loss of gut microbes differed between infected immunocompromised and WT mice, and whether this was linked to the difference in the brain pathology seen in these two groups.
The gut microbiome
Researchers examined the composition of gut microbiota, using faecal samples. They demonstrated that, whilst infection caused no change in species richness in the immunocompromised vim−/− mice, a significant decrease occurred in infected WT mice. This included a reduction in several species of Lactobacillus that play an important role in immune regulation and the maintenance of gut epithelial integrity.
In an attempt to reverse this reduction in gut bacteria, two species of Lactobacillus were repeatedly transplanted into WT mice by gavage. Both bacterial species significantly improved the survival of the infected group and decreased the burden of T. gondii cysts. Damage to the intestinal barrier, brain inflammation and neuronal apoptosis was mitigated.
The authors attributed the decrease in parasite cysts, in part, to an increase in the proportion of circulating CT8+T cells and of serum IFN-γ, both important components of the immune system that are indirectly activated by Lactobacillus-related metabolites, including indole-3-lactic acid (ILA), which is reduced in T. gondii infections.
In summary
The authors of this very thorough study concluded that pathological effects resulting from T. gondii infection are exacerbated by the response of the host immune system to infection, as they were more severe in WT mice than in immunosuppressed ones.
This host-immune response disrupted the integrity of the gut and damaged the brain because the gut microbiota was adversely affected.
Inflammation of the gut, damage to the intestinal barrier and neuronal apoptosis improved when depleted probiotic lactobacilli were replaced.
One of the key bacterial metabolites that was downregulated was ILA. The researchers suggested ILA could be a useful therapeutic compound to treat T. gondii infections. If successful, it could be easier to administer than transplantation of Lactobacillus strains.

Comments