We’re just past the season of graduations. This was especially important for me personally this year, as my son has just graduated high school. One of the values highlighted at his graduation ceremony was gratitude, for all the hard work students put in over the last four years. This made me think of the students who I had the privilege to work with in the latest round of my Disease Ecology capstone class this Spring quarter. This was a cohort of students who started college right before or during the start of the COVID-19 pandemic, in 2020. They had to endure and adapt to the upheavals and demands of that time – from having to learn through Zoom in their first year or two, to having to risk their health and lives by working as “essential workers”, often in healthcare settings.
Having worked with them this Spring, I’m both grateful for their spirit, perseverance and contributions, and very hopeful for the next generation of biologists. I’m grateful to them for trusting me with their last quarter at EWU and choosing to take their Senior Capstone class with me. As I explained it to them, my approach in my Disease Ecology class (as you can see in previous posts over the years) can be defined as organized chaos. Beyond going through and digesting together the chapters of the book Infectious Disease Ecology and some other readings, students work together in groups to research, design, run, analyze, document and present a project of their own choice on a One Health-related topic. They chose projects ranging from collecting ticks and mosquitoes, through testing dogs, fish and bats for infections, to detecting antimicrobial resistance in soil bacteria. Given the 10-week length of our quarters, they did not have much time for any of this. They had to choose their topic in the first week in April, research and design their projects and present a proposal on them by the beginning of May, get their supplies and conduct their study by the beginning of June, and present a poster and submit a scientific article by the middle of June. As anybody who has done research knows, this is hardly enough time – however, that’s all they had. I kept reassuring them that it was all going to work out in the end, and they had to trust me in that. And as usual, it did all work out at the end.
However, there were plenty of frustrations and complications to overcome in the meantime, which they weathered very well. Some of the projects required permits, some multiple, given that they dealt with vertebrate animals, and some on federal lands. Since proposals were still being finalized at the beginning of May, it took a couple of weeks to obtain permits. Meanwhile, the students weren’t able to collect their data and had to rush to get everything done at the end when they got the permits approved. In the canine project, permits were obtained just during the last week, and supplies had to be ordered which arrived just in time to conduct the testing as the permit was obtained. For the antibiotic resistance group, there was an issue with the calculation of antibiotic dosage into the agar plates that were made, which meant they had to make new plates with different dosage and try again. For the mosquito group, just as they were about to start collecting in earnest, the previously balmy spring weather turned cold and windy, not really conducive for mosquito collections. However, they were all able to overcome these obstacles, and produce quality (if sometimes inconclusive) data and conduct a study they could be proud of.
The best example of their adaptability (that I know of) happened in the canine group. They were looking for intestinal worm eggs in canine feces using the sugar-flotation method in the lab one evening after sample collection. They had access to our classroom lab and a centrifuge in a different lab, but not to our stockroom, and no-one else was around to let them in there. When they needed to strain the slurry before centrifugation, they realized that they did not grab an appropriate filter from the stockroom previously. They could have easily given up at this point, just gone home, and asked me what to do next. However, they did not do that. Instead, they had the ingenuity to use the gauze from the first aid kit in the lab as a makeshift filter, and it worked! They were able to centrifuge the filtered slurry and collect intestinal worm eggs (along with some pine pollen) on their slides and estimate the prevalence of worms in the canines they studied.
These, and similar stories, make me very hopeful about this upcoming generation of biology professionals. Students in my class took ownership of their projects, worked on them passionately, and were proud of their achievements. Most of them seemed to appreciate and value the importance of One Health concepts discussed in class, such as the importance of preserving biodiversity, including the important role pathogens and vectors play in ecosystem processes. Despite having come from varied backgrounds and going off to various career trajectories (all the way from agriculture to dental programs), they saw the connections to their own fields, and the relevance of their class content, including through visits from alumni working in public health. Finally, they appreciated the connections and importance of their projects to the community, working together with staff from animal shelters to local, state and federal agencies.
Perhaps I got lucky with the students enrolled in my class this Spring, but they give me hope for the next generation of biology professionals. I’m looking forward to seeing how far they will come and what they will achieve as they carry on with the same enthusiastic and hard-working attitude that they showed in my class. For now, I’m just grateful to having had the privilege to work with them this Spring.
For short summaries of their projects, please see below!
Questing Tick Population Density in Spokane, WA Parks
Ashley Babin, Xochi Chavez, Joshua Estudillo, Jacob Heaton, Nayeli Hernandez

Ticks are the leading vectors of disease in the United States. While tick-borne diseases are relatively rare in Washington State, Spokane County has experienced unusually high levels in the past. Our study aimed to investigate the distribution and density of ticks along popular hiking trails near Spokane, WA, focusing on areas with high human-tick contact potential. We conducted tick drag sampling along four trails: Bowl & Pitcher, Dishman Hills, Rocks of Sharon, and Indian Canyon Mystic Falls. Each trail was divided into three 100-meter sections, further broken into ten 10-meter transects adjacent to the trail edge. Ticks were collected using a 1 m² corduroy sheet and preserved in 70% ethanol. Our results showed that there were no significant differences in tick population densities among the four trails studied. In contrast, the Turnbull National Wildlife Refuge exhibited significantly higher tick densities. Both Dermacentor variabilis and Dermacentor andersoni species were present at Turnbull, while only D. variabilis was found at Indian Canyon Mystic Falls and D. andersoni at Rocks of Sharon. Vegetation type did not significantly affect tick distribution. These findings suggest that while most hiking trails near Spokane pose low tick-borne disease risk, precautions are advised for trails like Indian Canyon Mystic Falls and Rocks of Sharon, where ticks were present. Extreme caution is recommended at Turnbull National Wildlife Refuge due to its high tick population.
Stealth Spread of Canine Diseases at a Spokane Animal Shelter
Anna Carroll, Ana Beatriz Granman and Colton Quinn

The purpose of this project was to look for two very common canine pathogens, canine influenza and canine intestinal worms. Through two different testing methods many animals at the local animal shelter were able to be tested for both of these pathogens. Once the number of canines infected were analyzed we were able to determine the prevalence of the two pathogens. We found worm eggs in 3 out of 7 fecal samples and identified them as Ancylostoma hookworms. Also, 4 out of 20 dogs tested positive for canine influenza. Using this information, we were able to help advise the staff of the animal shelter in what we would think would be some beneficial changes in order to help prevent the spread of further cases among their canine population.
An observation of bat microbiomes and ectoparasites in Eastern Washington
Jackie Luna, Chelsea Schur, and Makenna Tabino

In this project, we worked with the Washington Department of Fish and Wildlife (WDFW) and the Bureau of Land Management to observe disease in a known maternity colony. The WDFW were testing for a fatal fungal infection called White Nose Syndrome, caused by the fungi Pseudogymnoascus destructans. We decided to swab and culture bacteria and fungi that grow on the bat’s wings and collect any ectoparasites observed on the bats to see

if there were any correlations between these three factors. We were unable to discover any definitive results as we were unable to collect a sufficient amount of samples due to a declining population, and we also were unable to receive results on the WNS testing in time. However, with our limited data, there was a positive correlation between the microbiome’s robustness and ectoparasites’ presence. We also discovered a previously unobserved bat tick in the colony but could not identify what family or species it belonged to due to its size and our limited technology. By observing these different elements of a bat’s body ecosystem, we can attempt to identify what causes a bat to be susceptible to WNS. We can also observe how a bat’s microbiome and ectoparasites coexist together.
Impacts of Antibiotic Pollution on the Density of Antibiotic Resistant Bacteria in Greater Spokane, WA
Marion George, Alex Hays, Aspen Johnson, Ilona Kutsar and Raegan Laycock

The spread of antibiotic resistance genes is a large global health threat to the mitigation of bacterial infections. Extensive use of antibiotics, wastewater treatment, agricultural practices, and pollution has encouraged the propagation of antibiotic resistance genes and antibiotic resistant bacteria. Our study aimed to provide insight into the differential densities of antimicrobial resistant bacteria throughout Spokane, WA through sample collection from areas of variable antibiotic pollution. We suspected more populated areas will have higher concentration of antimicrobial resistance due to mammal excretion of unmetabolized antibiotics in sewage, pharmaceuticals, and personal care products into water runoff. To test this, we collected soil samples from locations we expected to have antibiotic pollution (near Sacred Heart Hospital, Long Lake, and the Spokane River) and locations we expected to have less antibiotic pollution (Turnbull Wildlife Refuge, Manito Park, and EWU Prairie Restoration site). We then ran three trials per location, diluting the samples and growing them on control TSA plates without antibiotics, as well as Ampicillin, Streptomycin Sulfate, and Chloramphenicol spiked TSA plates. We calculated colony forming units (CFUs) and percent of antibiotic resistant bacteria at each location and ran both ANOVA and Logistic Regression statistical analysis on our data. Although we didn’t find a significant difference in the antibiotic resistance between specific locations, we did find that location was a significant predictor for antibiotic resistance. We also found a significant difference in the probability of there being Ampicillin resistance over Chloramphenicol resistance near both Long Lake and Sacred Heart, which hints to the fact that Ampicillin is more widely used than Chloramphenicol. This research paints a more complete picture of antibiotic resistant bacteria density in the Spokane area and provides a better understanding of the spread of antibiotic resistance due to antibiotic pollution and the need to slow this pollution.
Detection of a Well-Established Parasite within Invasive Brook Stickleback (Culaea inconstans) Population at Turnbull National Wildlife Refuge
Johnny Cardenas, Dalton Dawson, Flavio Rosales, Jessica Smith, Vladis Zetchov
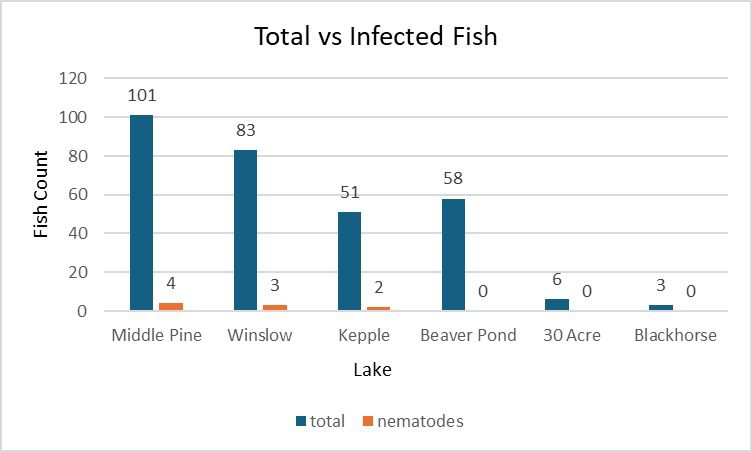
Our study at Turnbull National Wildlife Refuge (TNWR) looked at the presence of the parasitic nematode Contraceacum multipapillatum in invasive Brook Stickleback populations across six ponds and lakes. We found varying infection rates, with Middle Pine Pond having the highest (3.96%) and some sites, like Beaver and 30 Acre ponds having no infections. We thought there would be a link between fish size and nematode presence, but this was proven wrong by calculating the Ponderal Index (PI), which is like BMI. Delays in obtaining permits and limited time affected our data collection, highlighting the need for further research. Our findings suggest a wider distribution of Stickleback than what was previously known.
Mosquitoes: One of the World’s Most Dangerous Insects
Braelyn Ballou, Jonathan Becerra, and Hunter Briner

When you think of dangerous animals, you think about lions or bears. However, mosquitoes can be super dangerous to humans and animals in general due to the many diseases they can carry. Mosquitoes are vectors for diseases like Malaria, Yellow Fever, and West Nile virus which was found in Spokane County a few years ago. WNV is carried by Culex pipiens, a species of mosquito found in Washington State. Our project was designed to be able to identify mosquito and mosquito-like insect breeding sites so later projects can trap these locations to track the movement of diseases and mosquitoes. CDC light traps were used to trap adult insects and water samples were collected to identify any larvae in the water to see if the adult insects were breeding in those locations. We compared both larvae and adult insects captured to water pH and other variables to see if the mosquitoes and mosquito-like insects select specific habitats. Our results were inconclusive, and we did not trap enough mosquitoes due to the weather and temperature being cold and windy. However, water pH statistics showed to be significant in one area which opens the door for more experiments in the future to see if that statistical value stays significant in other ways too. Overall, our experiment can help to identify variables and breeding sites for these insects for future monitoring in Spokane County and to track WNV in mosquitoes and other diseases in mosquito-like insects too.

Comments