
Apicomplexans (Apicomplexa) are a large and diverse phylum of single celled parasitic organisms that includes many medically and veterinary important pathogens, such as the gregarines, coccidians, cryptospordia, and plasmodium species. Parasites in these groups cause severe disease in humans, as well as wild and domesticated animals.
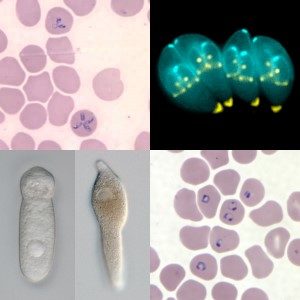
For example, species in the genus Plasmodium cause malaria in animals and humans in over 90 countries worldwide, and according to The World Health Organisation, are responsible for approximately 450,00 human deaths annually.
Currently, no effective vaccine exists for many of the diseases caused by these parasites, and control and treatment of infections has demonstrated to be a challenging task. For instance, Toxoplasma gondii can infect every known mammalian species, establishing an untreatable and dormant infection, which, when activated in pregnancy can result in severe birth defects. Plasmodium species on the other hand are remarkably ingenious parasites, perfectly adapted for evading the hosts immune system and rapidly developing drug resistance. Due to challenges such as these, the identification of novel compounds and the development effective drugs are crucial in controlling and preventing these parasitic infections, in animals and humans alike.
A hunt for anti-apicomplexan compounds
A recent study aimed to identify new compounds with anti-apicomplexan activity by hypothesising that symbiotic bacteria found in invertebrates may be an abundant (and natural) source of these anti-apicomplexan compounds.
Just like mammals, invertebrates are susceptible to infections with gregarines. Gregarines also happen to be ancestral to all apicomplexans, implying that an anti-gregarine compound produced by this invertebrate-bacterial symbiosis may also be effective against many of the other apicomplexans plaguing hundreds of millions of humans and animals globally.
In this study, Roberta O’Connor and colleagues decided to investigate a bizarre bacterial symbiosis in shipworms; a marine bivalve mollusc in the family Teredinidae.

This family contains an unusual collection of long, soft-bodied clams that burrow into and ingest saltwater-immersed wood. However, they cannot digest the wood themselves, and therefore rely on intracellular bacteriapicomplexana to complete the process. These symbiotic bacteria were also found to produce compounds that likely play a role in protecting their host’s gut (and therefore themselves) from pathogens. This, combined with the knowledge that shipworms can be infected with gregarines, provided the rationale that this invertebrate-bacterial symbiosis could prove to be a prosperous source of novel apicomplexan therapeutic agents.
Testing for anti-apicomplexan activity
Two strains of the symbiotic bacterium found in the gills of shipworms; Teredinibacter turnerae (T7901 & T7902) were extracted from shipworms, artificially cultured, and subsequently screened for activity against Toxoplasma gondii in an attempt to identify specific anti-apicomplexan compounds. T. gondii was then allowed to infect human fibroblasts and then treated with both bacterial symbionts. Parasites treated with supernatant from T7901 showed rapid growth inhibition, aggregation within the parasitophorus vacuole (PV), expansion of the PV within human host cells, and ultimately, parasite death after 2 hours of treatment. Twenty-four hours after treatment no morphologically intact parasites could be observed within the PV. Supernatant from T7902 had no effect.
The active compound
Upon further investigation, the researchers discovered that a compound called Tartrolon E, or Trte (an ionophoric compound with antibiotic properties), was responsible for the strong anti-T. gondii activity demonstrated.
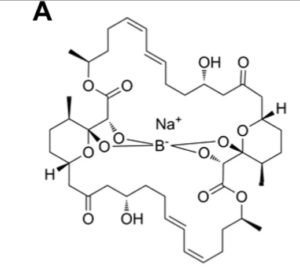
It was discovered that Trte killed the parasites strikingly quickly, even at nanomolar concentrations, was highly specific in targeting only parasite cells (and not host cells), and most importantly, was effective against all tested apicomplexans (Babesia bovis, B. bigemina, Theileria equi, Plasmodium falciparum, Cryptosporidium parvum and Sarcocystis neurona).
The broad spectrum activity of Trte displayed likely means that it exhibits activity in all branches of the apicomplexan phylogenetic tree, and could therefore be a new and effective therapeutic drug against a range of apicomplexan-related medical and veterinary disease, something which has never been demonstrated before. After searching only two symbiotic bacterial strains in this one strange organism, the identification of a new, efficacious compound such as Trte is highly promising, and should encourage others to further investigate invertebrate symbionts for new anti-parasitic therapeutics. This is undoubtably an exciting time for anti-apicomplexan drug development.

Comments