Antimalarial drugs
The historical relationship between the malaria parasite and drug treatment has always culminated in the development of drug resistance. Although once considered the ultimate weapon against Plasmodium, evidence of resistance to artemisinin-derived drugs has already emerged, making the discovery of novel drugs and drug targets imperative. A good antimalarial should target a fundamental process of parasite biology and be non-toxic for the human host (we are pragmatic).
One of the main features of the malaria parasite (Plasmodium) life cycle is the multiple cycles of invasion, multiplication and egress in the human red blood cells which cause the characteristic symptoms of the disease such as cyclic fever. Evidently, targeting the egress/invasion machinery, at a point when the parasite is most vulnerable (not protected within host cells), could have a huge impact not only on disease severity but also disease transmission.
Egress and Invasion
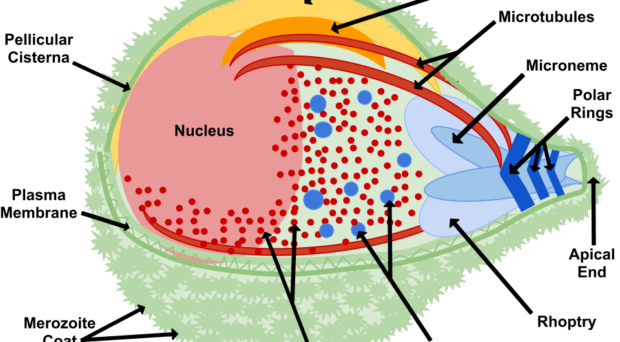
Once in the red blood cell the parasite matures from a ring to a trophozoite and finally a schizont, producing 16-24 (for P. falciparum) merozoites primed for invasion. The release of merozoites, known as egress, is a two-step process; the degradation of the parasitophorous vacuole (which encloses the merozoites) and red blood cell membrane. Invasion of a new red blood cell takes only 10-30 seconds and involves recognition of the red blood cell membrane, attachment, reorientation and entry (for a detailed review of this process, please refer to a review by Alan Cowman and colleagues). Many of the proteins involved in invasion and egress are packaged into two main secretory organelles in the merozoite, the rhoptries (invasion) and the exoneme (egress).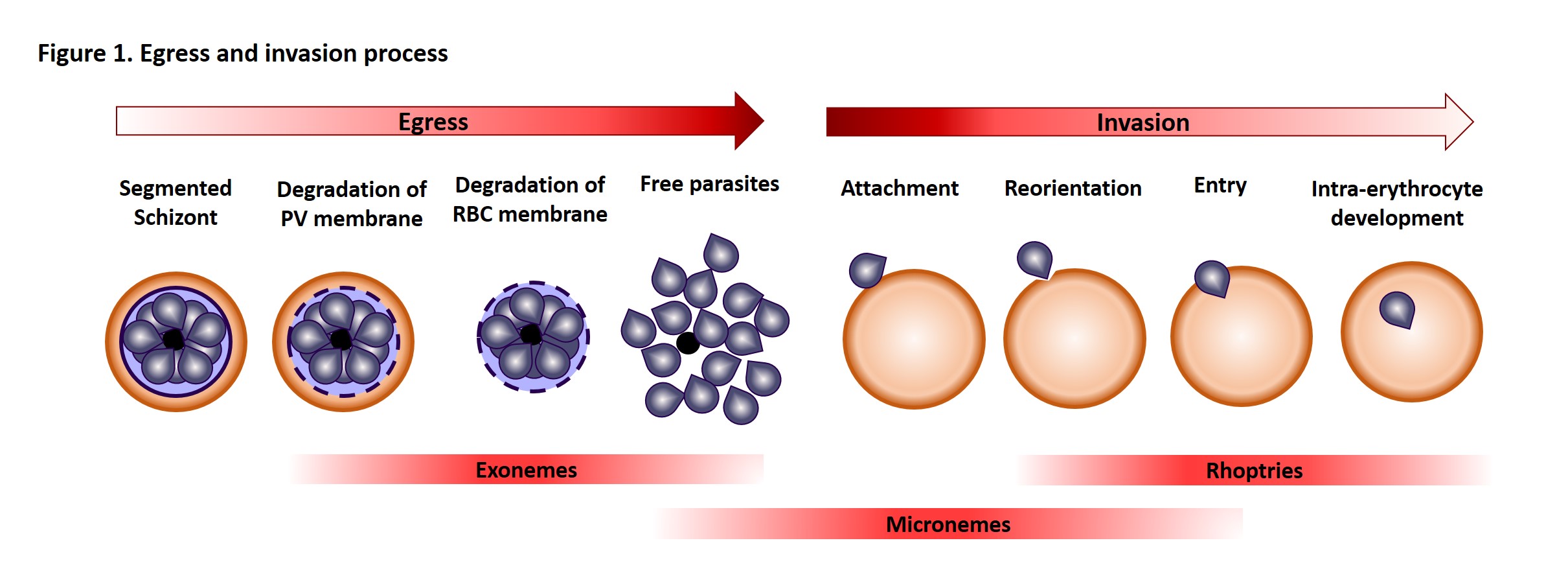
Interestingly, rhoptries and exoneme are not restricted to Plasmodium species but can also be found in other apicomplexans, where they have a similar role in invasion process (for more details, see the review by Tobili Sam-Yellowe). Thus, making discoveries in the malaria parasite is relevant to other parasitic diseases.
Plasmepsins
Plasmepsins are a group of Plasmodium specific, aspartic acid protease enzymes, of which there are 10 isoforms (PMI-X). PMI-IV are found in the digestive vacuole and are not essential to parasite survival. PMV, PMIX and PMX are the only other plasmepsins expressed in the red blood cell stages. PMV is essential and is already a focus of drug development. PMIX and PMX have been shown to be expressed in late schizont/merozoites, and their potential involvement in egress-invasion processes has been the focus of two recent studies by Paco Pino and colleagues and Armiyaw Nasamu and colleagues. Both studies, which are the subject of this blog, used similar approaches to characterize PMIX and PMX, supporting their validation as drug targets and presenting two novel inhibitors 49c and CWHM-117.
Conditional Knock down and chemical inhibition
One of the most straightforward ways to study the importance of a specific protein is to remove it and see the consequence (like Brexit!). Since both groups failed to generate knockouts, inferring there were essential functions for the proteins, they used conditional knockdown (KD) approaches that block protein expression under specific drug treatment. Both teams generated a KD for PMIX and Pino and co-workers generated a PMX KD. Interestingly, when PMIX is not expressed, the parasite can egress as usual but was less able to invade new erythrocytes. The absence of PMX, on the other hand, leads to a decrease in egress and a further decrease in invasion. The double KD had a similar effect on egress but a 7-fold block in invasion. Obviously, such features are desirable drug targets.
Summary of findings
| What is worth keeping? |
| PMIX is involved in invasion
· Conditional KD develop normally into segmented schizonts, egressed normally but there was only 25% of ring stage parasites in newly invaded erythrocytes compared to controls · Tagging localised PMIX to the rhoptry · Not expressed in gametocyte (no need to invade new erythrocyte at this stage anyway!) |
| PMX is involved in invasion and egress
· Conditional KD prevented parasite egress as well as new invasion (of those that egressed only 50% invaded new erythrocytes) · Tagging localised PMIX to the exoneme |
The authors also tried to identify the targets of these proteases. Consistent with their localization PMX KD had reduced processing of the exoneme protein SUB-1 (PMIX KD no effect) and, predictably, the PMIX KD affected the processing of the rhoptry protein RAP1 (PMX KD no effect). The authors analysed a whole host of other exoneme, microneme, rhoptry and merozoite surface proteins in the PMIX and PMX KDs shown in the table.
| Protein | Phenotype | Localization | |
| PMIX KD | PfASP | processing impaired | Rhoptry |
| PfRAP1 | precursor accumulation | Rhoptry | |
| PfRH5 | no effect | Rhoptry | |
| PfRiPR | no effect | Rhoptry | |
| Pfp113 | no effect | Merozoite surface | |
| PfCyRPA | lower amount secreted | Microneme | |
| PfAMA1 | no effect | Microneme | |
| PfSUB1 | no effect | Exoneme | |
| PfSERA5 | no effect | Exoneme | |
| PfMSP1 | no effect | Parasitophorous vacuole | |
| PMX KD | PfAMA1 | cleaved by PMX | Microneme |
| PfSUB1 | processing impaired | Exoneme | |
| PfSERA5 | precursor accumulation | Exoneme | |
| PfMSP1 | precursor accumulation | Parasitophorous vacuole |
| The different proteins analysed after KD of PMIX and PMX |
Anti-plasmepsin drugs
As we have seen, the absence of PMIX and PMX blocks parasite development impairing egress or invasion of a new erythrocyte. Such features would make good drug targets. Both research groups tested an aspartyl protease inhibitor, 49c (Pinto group) and CWHM-117 (Nasamu group). They noticed egress and invasion phenotypes similar to those seen in the KD parasites. Interestingly, when 49c was removed before parasite egress, the parasite invaded new erythrocytes normally (point to keep in mind for future drug design). The 49c drug was also tested in vivo as well as on other Plasmodium stages (no restrictions when it comes to potential drug). They could see some efficiency against hepatocyte and gametocyte egress as well as interference with the processing of the CelTOS protein in the ookinete (all of this sounds good for a broad action antimalarial).
| Drugs targeting plasmespins block parasite development | |
| 49c | · Treatment is time-dependant (no effect if removed too early or added too late)
· Blocks parasite egress from erythrocytes and hepatocytes in vivo (a rodent malaria, P. berghei in mice) and ookinete or oocyst formation (if treated during gametogenesis) |
| CWHM-117 | · Blocked erythrocyte egress and invasion
· Combination of KD and inhibitor completely blocked egress · CWHM-117 can be taken orally (shown in mice, but this is a start) |
Conclusion
Through these two studies, the role of plasmepsins (IX and X) in the key event of getting out and into the erythrocyte by the parasite have been highlighted. The novel drug targets present an exciting opportunity to target multiple stages across the parasite life cycle. Two drugs targeting these proteins have shown interesting results, but the short time in which they have to act (between the parasite egress and the invasion of new erythrocyte) remains a tricky detail to address.

One Comment