
As defined by the International Committee of Journal Editors “the purpose of clinical trial registration is to prevent selective publication and selective reporting of research outcomes, to prevent unnecessary duplication of research effort, to help patients and the public know what trials are planned or ongoing into which they might want to enrol, and to help give ethics review boards considering approval of new studies a view of similar work and data relevant to the research they are considering”.
All through 2014, calls to further increase transparency of clinical trial data have continued to gather pace. From providing a space for public trial registration to facilitating deposition of basic results and links to peer-reviewed publications, the goalposts have been moving and expectations have continued to increase:
- Alltrials continue to campaign for “all trials to be registered and all results to be reported”.
- The UK’s Health Research Authority is linking ethics approval to registration and, in 2015, is planning to go one step further, by asking study sponsors to declare that that all clinical trials in active recruitment in the UK have been registered. Ethics approval and study design information are essential in registries.
- The World Health Organization, credited with devising the minimum dataset that best describes a study, launched a consultation regarding the next logical step, the reporting of clinical trial results and is expected to issue a statement in early 2015. Registries should also include a section on results dissemination.
Throughout this period, we have consulted with a number of stakeholders and key opinion leaders in the field, and worked on re-launching the ISRCTN website to both meet those new expectations and anticipate any future requirements. We are still keen to hear your thoughts, more about this at the end.
The ISRCTN registry was originally created in 2000 as part of the Current Controlled Trials platform, managed by BioMed Central, in response to the need for more transparency around clinical trials, expressed by a number of organisations including the UK Medical Research Council.
However, a major revamp was long overdue, because of the now outdated software behind the website, and because of multiple names that had ended up muddying our key mission: A science publisher aiming to maximise dissemination by providing a freely-available platform for what is seen as the first step toward transparency in health research and clinical trials.
The new website was switched live on 19 November 2014. We have simplified our name – we are the ISRCTN registry – and our website has a new, modern look and logo, and greatly improved functionality.
The main features are as follows:
- We have made it easier to submit study details – researchers can now save submissions in progress and track progress of their applications. This appears under the my trials section when you are logged in.
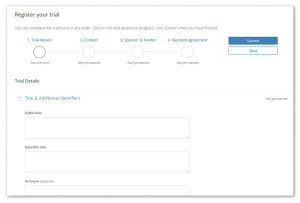
- More information is collected such as recruitment start and end dates and participating sites – that will ultimately allow prospective participants to find research that is directly relevant. See this example.
- When available, a Plain English summary is always displayed at the top of a record. This helps non-experts and potential future trial participants to get a quick understanding of what the research is about.
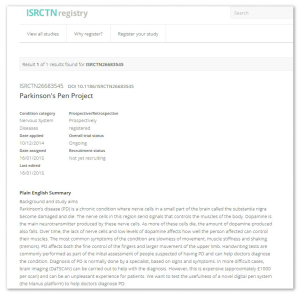
- We have improved our results and dissemination section to accommodate forthcoming requirements on basic result reporting and to allow researchers to declare their position with regard to future sharing of raw (or participant level) data. An example can be seen here.
- The site now has an advanced search.
- A very straightforward fee payment system has been implemented – this can reduce the time from editorial acceptance to public listing.
- The website has been mobile optimised for use with smart phones and tablets.
- Active twitter account that keeps you informed of new studies registered, publications added and other news.
This is a new start for the ISRCTN registry, which helps us strengthen BioMed Central’s commitment to public engagement in research, also demonstrated by the launch of a new type of journal, co-produced by both health professionals and lay members: Research Involvement and Engagement.
We are inviting all of you to tell us what you think of the new ISRCTN registry site by filling in our survey. There is still a lot to be done to ensure that the registry keeps on meeting evolving expectations – do not hesitate to contact us with any questions or comments.
Comments