
The last few years have been significant for trial registration, as the role it plays in research is recognized by global initiatives and journal publication requirements. And with good reason; trial registration is paramount in research transparency, minimizing bias and selective reporting, as well as publically showcasing what research is currently being undertaken to channel valuable research funds and efforts where needed, avoiding duplication.
In 2008, the Declaration of Helsinki was revised to state “Every clinical trial must be registered in a publicly accessible database before recruitment of the first subject”. This is prospective registration (as opposed to retrospective registration, which is registration after the first participant is enrolled); the policy is widely known and many journals require or encourage it, but are we seeing this reflected at the registry level?
Ten years of mandatory trial registration – what does the data say?
We looked at registry records in the ISRCTN Registry*, which is curated and administered by BMC, to investigate trends.
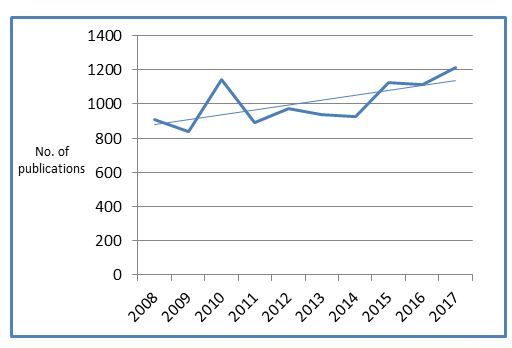
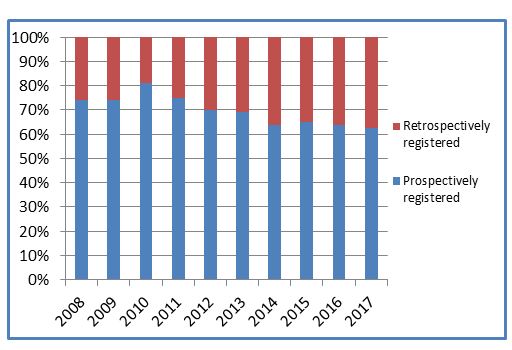
While absolute numbers of ISRCTN records are continuing to grow, we are not seeing the same with prospective registration. Interestingly, despite a peak in 2010, over the last four years it has remained relatively stable, with just under two-thirds of studies registered prior to starting recruitment.
Although prospective registration remains the ultimate goal, perhaps in reality the current message is better late than never (a full discussion on the Editors’ dilemma of retrospective registration is here).
The momentum of campaigns like AllTrials and Restoring Invisible and Abandoned Trials (RIATs), which urge researchers to register and publish past and completed trials, continues to grow. Are we seeing this slight decrease in prospective registration as the backlog of old trials are being registered and published, or is something else at play? It’s also interesting to consider whether requiring prospective trial registration at the time of submitting to a journal is just too late in the process.
An evolutionary platform
It’s not just about numeric growth; as submissions increase, ISRCTN provides an adaptable platform that is constantly evaluated and updated to keep up with guidelines and the progression of the clinical trial field as a whole.
Digital object identifiers (DOIs) help to connect all resultant publications to the trial record, irrespective of publisher, and increase the permanence of digital publications.
Have you ever considered participating in a trial? Clinical research would not happen without patients and volunteers! In 2011, ISRCTN recognized the importance of this, becoming the data source for the launch of UK Clinical Trials Gateway, a Department of Health initiative to encourage trial participation. We added a plain English summary to each ISRCTN trial record, to make current research more accessible and understandable to those who play such a crucial part.
2012 saw digital object identifiers (DOIs) added to all records, helping to connect all resultant publications to the trial record, irrespective of publisher, and increase the permanence of digital publications.
ISRCTN underwent its biggest revamp to date in 2014, when the site re-launched. This improved usability and incorporated new fields, such as ORCID IDs (a unique identifier directly linking trials to researchers) and publication plans. A significant update introduced here was the retrospective/prospective flag system, automatically generated using compulsory recruitment start and end dates to clearly label a trial’s registration status, as this developed as an increasingly important factor for publication.
More recently in 2017 ISRCTN advanced in accordance with four new items added to the WHO data set, encouraging trialists to share ethical approval details, basic results prior to publication, and their plans around data sharing.
Collaboration is crucial
Clinical trial conduct is a vast, progressing field that would be almost impossible for ISRCTN to stay on top of alone! Relationships with external partners, other registries and global initiatives provide guidance and support in registry development.
A key partnership is with the National Institute for Health Research (NIHR), a UK government body that coordinates and funds research for the National Health Service (NHS). As a significant funder of health and care research in the UK, the NIHR funds an increasing number of records published in ISRCTN. Matt Westmore, Operations Director at NIHR Evaluation, Trials and Studies Co-ordinating Centre, recognizes that “working in partnership is a much more powerful and efficient way of realising the value of registries.”

As lead on the Adding Value in Research initiative, which won a 2017 Cochrane-REWARD prize for reducing waste in research, Matt considers the importance of registries as this:
“Lack of registration is a major source of waste when in effect the same trial is repeated because the results are not known, and because the world doesn’t know it’s on-going. Registration is important to prevent unintentional duplication and provide an audit point for later accountability. What’s particularly important is that registries are about on-going studies, because there’s almost nowhere else you can find out about that, but it’s also about what has happened, as so many trials go unpublished. Often the only place that there’ll be a record that a trial ever existed is within a clinical trial registry.
Lack of registration is bad for science and bad for patients.
This doesn’t just apply to clinical trials. In the future we’d like to see greater linkage and interaction across the research pathway, across different research methods and across different registries- it needs to become a much more coordinated and consolidated view.
Ultimately we want to be able to say that all studies are registered in an appropriate publicly accessible registry at study inception and easy to find through simple searches. It is not just NIHR that thinks that. An international forum of funders has agreed this as an important guiding principle.”
So, what’s on the horizon for ISRCTN?

To round off, new ISRCTN Database Manager, Claire Veryard, shares some of her reflections on ISRCTN since joining the team in March this year:
“What has struck me about ISRCTN is how varied the trials are – ISRCTN has the flexibility to accommodate any type of intervention, observation or trial design. I’d like to improve the search and output functionality of ISRCTN so that it is easier for researchers to check for similar trials and to compare designs, as well as distinguish between trials with and without results. We also have plans to develop more thorough publication summaries.”
Enjoyed our post on trial registration? Explore some more hot topics that we have collated as part of Clinical Trials Day 2018 here!
Want to test your knowledge of trial registration? Take our ISRCTN quiz.
*The ISRCTN Registry is an open access, freely accessible and searchable clinical trial registry, Open to any study designed to assess the efficacy of health interventions in a human population, ISRCTN ensures trial records contain the essential information required by the World Health Organisation (WHO).
Comments