What is dementia with Lewy bodies?
Dementia with Lewy bodies (DLB) is a progressive neurodegenerative disorder that typically results in changes in attention, speed of information processing, visuospatial/visuoperceptual capacities, planning and memory.
Cognitive functions typically fluctuate,with episodic confusion and reduced vigilance. Recurrent visual hallucinations, and parkinsonism are the other defining clinical features of DLB, in addition to which the patient can ‘act out his/her dreams’ while sleeping (rapid eye movement sleep behavior disorder, RBD).
Among more than 60 millionin habitants in France, one million people have dementia, and of those, 200,000 will have DLB. DLB differs from Alzheimer’s disease (AD) both in the cognitive pattern – patients with AD typically have predominant memory impairments – and also by the absence of the DLB core clinical features in AD.
Diagnosing the disorder
Patients may not mention the tell-tale hallucinations to their clinician, not least because the clinician does not always ask about them.
The differential diagnosis between the two disorders is however not so easy in clinical practice. Patients may not mention the tell-tale hallucinations to their clinician, not least because the clinician does not always ask about them.
Similarly, patients and families are not easily able to describe the cognitive fluctuations to their doctor, and even if they do, the diagnostic significance of these isn’t always recognized. Parkinsonism is not always present in DLB, and even when it is, it can be so mild that it is hardly distinguishable from normal aging.
Patients may also be unaware of their RBD symptoms, especially if they sleep alone and there is no witness. The sleep problem may have been present for many years before cognitive decline, and the patient and their family do not connect the two phenomena, as indeed the clinician may not, unless they have specialist knowledge of how DLB can present.
These diagnostic difficulties are even more problematic at the stage of mild cognitive impairment (MCI)/prodromal DLB.
A case example
To better explain, we can use the case of an 83 year old patient
At the beginning, the patient came alone and complained of memory impairment, but no other problems. The neuropsychological tests showed clear verbal and visual memory impairment,and subtle attentional deficits, without any other cognitive abnormality.
Global cognitive functioning was normal with an MMSE (Mini Mental Status Examination) score of 29/30. There was no parkinsonism on physical examination. Brain MRI showed only subtle hippocampal atrophy.
The diagnosis made was of mild cognitive impairment due to AD. No treatment was started but we followed the patient twice a year. We asked him about fluctuations, hallucinations, RBD every year, and the answer was always no.
The only surprising thing we detected during the follow-up wasclear variation of the MMSE (from 29 to 18, and then to 26, etc…), without any overall decline in functional capacity, the patient remaining independent at home.
Thanks to his partner who attended the clinic and explained to us that he has for two years had visual hallucinations of isolated animals, clear fluctuations with episodes of confusion and was one time agitated during sleep and kicking her in bed.
Finally we now have the answer, thanks to his partner who attended the clinic and explained to us that he has for two years had visual hallucinations of isolated animals, clear fluctuations with episodes of confusion and was one time agitated during sleep and kicking her in bed.
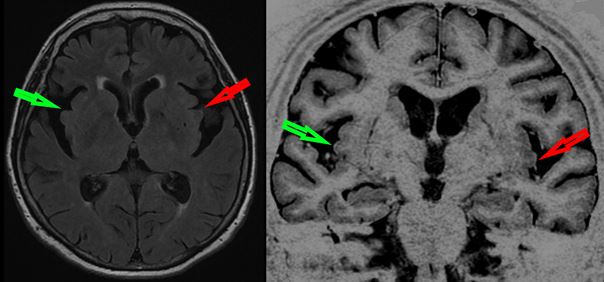
A few months before, we had decided to do a lumbar puncture for Cerebro-Spinal Fluid analysis and all the biomarkers of AD (Abeta42, Tau and Phospho-Tau) had been returned as normal. On repeated brain MRI, hippocampal atrophy was the same as seven years earlier, but when we looked more precisely at the insula, we saw greater bilateral atrophy that we hadn’t detected originally, but which on review had been there.
The reason for this is that previously we hadn’t been looking for atrophy of the insula. We only started doing it in Newcastle-upon-Tyne when we began a collaboration between Strasbourg and Newcastle to investigate the newly emerging concept of prodromal DLB.
What did we do?
First we demonstrated that comparing patients with clinically defined prodromal DLB and AD showed two things in terms of cortical thickness: first the well-known thinning of the medial part of the temporal lobes in prodromal AD patients, and secondly, the unexpected thinning of the anterior part of the insula in prodromal DLB patients. At the same time, colleagues from China described that insulae were bilaterally atrophic in a meta-analysis of seven cohorts of DLB patients.
Our next step, using the same patients from Strasbourg and Newcastle, was to study the focal atrophy of prodromal DLB patients using voxel based morphometry (VBM), finding the insula and anterior cingulate atrophy that we report here.
This is consistent with what we had demonstrated previously using the ADNI cohort when we compared a group of patients with hallucinations (which is probably the best clinical biomarker of DLB) and another with the same MMSE but without hallucinations. Here we could demonstrate atrophy of the right anterior insula in the hallucinators.
We recently found also that patients with prodromal DLB have a diminished perfusion of the insula.Thus, all these studies are consistent and in favour of early involvement of the insula in DLB.
The insula is a key region for cognition, emotions and neurovegetative aspects which has been implicated in the genesis of both hallucinations and cognitive fluctuations. We propose based on our findings to date that the insula is likely to be a specific marker of focal atrophy in DLB which will be a useful diagnostic marker differentiating it from AD, and applicable at the prodromal stage when clinical diagnosis is most difficult.
Comments