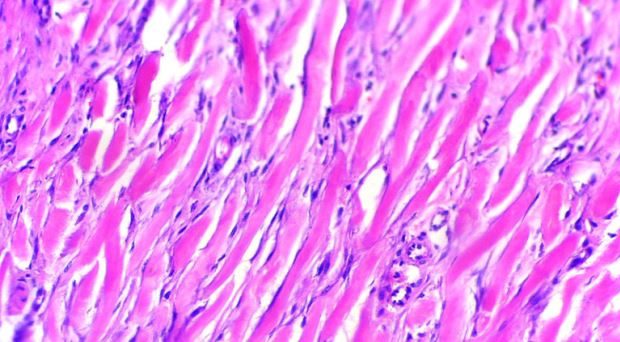
What are keloid scars?
Keloid scars are abnormal, fibroproliferative scars that can occur after burns or other skin injuries. Unlike hypertrophic scars, which represent a different form of abnormal scar, keloids extend beyond the original wound boundary. They are often refractory to treatment and have a tendency to recur, even after surgical excision. Keloids may continue to increase in size indefinitely, although unlike cancerous tumors, metastasis is not observed.
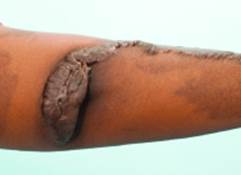
Keloids occur in all ethnic groups and in patients of all ages; however, they are significantly more common in patients with dark skin pigmentation, such as African Americans, and are most often observed during the second and third decades of life.
Keloids may run in families, or may occur spontaneously, and genetic studies suggest multiple modes of inheritance. Although multiple loci have been linked to keloid scarring in specific populations, no single causative gene has yet been identified, and it is likely that multiple genes, or multiple combinations of genes, are involved in susceptibility to keloid formation.
The molecular basis for keloid scarring remains incompletely understood. Because keloids do not form in animals, research has been hindered by the absence of an animal model. Historically, cells cultured from keloid biopsies have been an important tool for investigation of keloid pathology.
Keloids are characterized by excessive deposition of extracellular matrix. For this reason, fibroblasts have been the focus of the majority of keloid research performed to date. Keloid fibroblasts display numerous abnormalities compared with normal skin fibroblasts, including differential expression of growth factors relevant to wound healing and aberrant responses to growth factor stimulation. In particular, transforming growth factor beta 1 (TGF-β1), which has been associated with fibrotic changes in numerous tissues and cell types, is involved in the abnormalities observed in keloid fibroblasts. Other signaling pathways, such as the Wnt/β-catenin pathway, have also been implicated in keloid fibrosis.
Keloid fibroblasts display numerous abnormalities compared with normal skin fibroblasts
Keloid keratinocytes and epithelial-mesenchymal transition
While numerous abnormalities have been observed in keloid fibroblasts, less is known about potential involvement of keloid keratinocytes. Previous work from the laboratory of Dr. Dorothy Supp, at the Shriners Burns Hospital in Cincinnati, Ohio, identified abnormalities in keratinocytes isolated from keloid. These cells displayed changes in adhesion, motility, and differentiation, and altered expression of numerous genes involved in a process known as epithelial-mesenchymal transition (EMT).
EMT is a process whereby epithelial cells acquire traits normally associated with mesenchymal cells. Shifts in gene expression patterns are observed during EMT, and the cells become less adherent and more motile. TGF-β1 has been shown to be a potent inducer of EMT in numerous cell types.
EMT is a normal process that occurs during development, where it is critical during organogenesis, and during wound healing, as epithelial cells migrate to close a wound. In addition, EMT is associated with cancer and, in particular, tumor metastasis. In metastatic lesions, EMT is often accompanied by basement membrane breakdown, which allows cells undergoing EMT to migrate to distant locations.
During normal wound healing, EMT is a reversible or incomplete process; cells revert back to their normal, epithelial phenotype upon wound closure and resolution of inflammation. However, it has been proposed that under conditions of prolonged inflammation, EMT can contribute to organ fibrosis.
keloid cells may fail to recognize and/or respond to stop signals
An article recently published by Dr. Supp’s laboratory in the journal Burns & Trauma further supports a role for EMT in keloid pathology and implicates TGF-β1 as an important regulator of EMT in keloid keratinocytes. The authors found that stimulation of normal keratinocytes with TGF-β1 causes their gene expression pattern to become more keloid-like, and inhibition of TGF-β1 signaling in keloid keratinocytes normalizes EMT-like gene expression patterns. However, unlike EMT observed in metastatic tumors, basement membrane disruption is not observed in keloid scars. This may help to explain why keloid lesions do not metastasize.
EMT-like changes in keloid keratinocytes may indicate that the cells are stalled in the proliferative phase of wound healing. For reasons that are not yet understood, keloid cells may fail to recognize and/or respond to stop signals that normally regulate the transition from the proliferative phase to the remodeling phase of wound healing. The result is an overhealing response that leads to a keloid scar.
Although additional research is required to elucidate the specific mechanisms involved, these studies suggest that therapies targeting EMT in keloid keratinocytes may help to suppress keloid fibrosis, and should be explored as potential treatments to combat keloid scarring.
Comments